| Author | Affiliation |
|---|---|
| Hjalti M. Bjornsson, MD | Eastern Virginia Medical School, Department of Emergency Medicine, Norfolk, VA |
| Charles S. Graffeo, MD | Eastern Virginia Medical School, Department of Emergency Medicine, Norfolk, VA |
ABSTRACT
The identification and appropriate management of those at highest risk for life-threatening anaphylaxis remains a clinical enigma. The most widely used criteria for such patients were developed in a symposium convened by National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network. In this paper we review the current literature on the diagnosis of acute allergic reactions as well as atypical presentations that clinicians should recognize. Review of case series reveals significant variability in definition and approach to this common and potentially life-threatening condition. Series on fatal cases of anaphylaxis indicate that mucocutaneous signs and symptoms occur less frequently than in milder cases. Of biomarkers studied to aid in the work-up of possible anaphylaxis, drawing blood during the initial six hours of an acute reaction for analysis of serum tryptase has been recommended in atypical cases. This can provide valuable information when a definitive diagnosis cannot be made by history and physical exam.
INTRODUCTION
Identifying “a textbook case” of anaphylaxis is something that most practicing emergency physicians are comfortable doing. When asked, treating physicians are likely to describe patients who presented with evidence of circulatory instability or collapse, mucosal edema and, in some cases, mechanical obstruction of the airway generally in the context of various initial cutaneous manifestations such as urticaria, angioedema, flushing or pruritus. In this paper, we will review a growing body of data indicating that there is more to making this diagnosis than generally is appreciated, and how diagnostic accuracy can be improved.
THE DIAGNOSIS OF ACUTE ALLERGIC REACTIONS
Currently, there is no universally accepted clinical definition of anaphylaxis. The diagnosis may be challenging as there is a large variability in presenting clinical signs and symptoms. In recognition of this, the National Institute of Allergy and Infectious Disease (NIAID) and the Food Allergy and Anaphylaxis Network (FAAN) convened a consensus meeting in July 2005 on anaphylaxis, which included representatives from 16 different organizations from North America, Europe, and Australia, to develop a clinically useful and universally accepted definition of anaphylaxis and provide guidance on the most appropriate management of anaphylaxis. These guidelines were published in 2006 and are currently widely accepted (Table 1). Despite a wide array of symptoms included in the clinical criteria, the consensus panel concluded that one in twenty patients is likely to be misdiagnosed. Clinicians are left with a considerable number of cases where there may be some doubt concerning the diagnosis.1

Studies on anaphylaxis report a range in incidence from 3.2 to 58.9/100,000 cases annually.2–5This wide range is likely a reflection of the variation in diagnostic criteria used and the paucity of a “gold standard”. Published studies reflect this variation with some strictly narrowing inclusion criteria to subjects with hemodynamic instability or mucocutaneous involvement while others use broader definitions making interpretation difficult.3,5–7
The difficulties in standardization of research on anaphylaxis appears to also translate into clinical practice. A survey of 11 emergency medical systems (EMS) revealed a highly variable incidence of anaphylaxis ranging from 0.04% to 3.4% of all ambulance runs with the majority of systems reporting less than 1%. Among those EMS agencies reporting on the use of epinephrine autoinjectors, such injectors had been used in 0.16 to 31.1% of the cases.8 A study comparing 21 North American emergency departments (ED) noted that the recognition of acute allergic reactions caused by food exposures was highly variable. It was also notable that the proportion of patients referred to an allergist or provided with a prescription for self-injectable epinephrine on discharge from the ED was 12 % but highly variable9 (Figure 1).
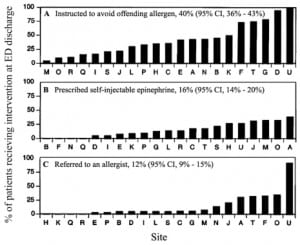
In one retrospective study reviewing all ED records for a period of four months only one in every four patients who met criteria for anaphylaxis had been correctly diagnosed during their ED visit.6The majority were diagnosed as having an acute allergic reaction. Based on the studies discussed above, there appears to be significant evidence of a need to standardize how anaphylaxis is diagnosed in the ED setting.
Beyond the complexities of identifying anaphylaxis, there are numerous reports of atypical presentations of acute allergic reactions that make this clinical diagnosis very challenging. This is evident in asthma, which is a disease related to anaphylaxis. In a study that reviewed the death records of individuals considered to have had an apparent fatal asthma attack, autopsy findings revealed that several of these patients had actually died from anaphylaxis.10 Acute symptoms of airway obstruction during an anaphylactic event have been misdiagnosed as being caused by a foreign body in the airway.11
Cardiac manifestations of anaphylaxis may mimic an acute coronary syndrome and acute anaphylaxis has been described to present with arrhythmias.12 This may be due to the physiologic stress of anaphylaxis in patients with pre-existing coronary disease, but histamine has also been shown to directly induce coronary vasospasm in individuals with normal coronary arteries.13,14This may occur as a part of the Tako-Tsubo or “broken heart” syndrome which is often attributed to abrupt physiologic stress.15,16 Exercise induced anaphylaxis may present as a syncopal event and should be considered in the differential diagnosis under appropriate circumstances.17
Vocal chord dysfunction is another cause of acute stridor presenting with a sensation of throat swelling. This condition can be challenging to differentiate clinically from anaphylaxis. When available, fiberoptic laryngoscopy during the attack will reveal no swelling but the characteristic intermittent adduction of the anterior vocal chords during inspiration.18–20 Other anxiety states such as panic attacks, globus hystericus and Munchausen stridor can also cause similar symptoms.
The symptoms of acute attacks of hereditary angioedema can be identical to acute allergic reactions but usually do not respond to medications used to treat anaphylaxis. Other conditions known to cause anaphylaxis-like symptoms are flush syndromes such as carcinoid or disulfiram reactions, restaurant syndromes such as reactions to monosodium glutamate, sulfites or scombroid, autonomic epilepsy and vasomotor rhinitis.
Considering all the above reported atypical presentations of anaphylaxis, it may be challenging in a significant number of cases to determine with any certainty the cause of a patient’s acute symptoms based on history and physical exam alone. This may be complicated even further if a detailed history is unobtainable or if a patient is not seen until after the acute symptoms have subsided.
LESSONS FROM SEVERE AND FATAL ANAPHYLAXIS
Fortunately, the majority of acute allergic reactions are mild and self-limited. Overall, mortality fromanaphylaxis appears to be relatively rare, on the order of less than 1% of all cases.2–5,7 The highest published mortality rates are in the pre-hospital setting with a Norwegian study reporting a mortality rate of 7%.21 However, several of the large anaphylaxis case series do not report a single case fatality.2,4–5,7,9 This may be due to selection bias since many of the fatalities occur in the pre-hospital setting. Published case series of anaphylaxis fatalities suggest an incidence ranging from 0.3 to 0.8 per 1,000,000 with an increase in fatality rates among the elderly presumably due to comorbid conditions and iatrogenic drug exposures.22–24 When acute anaphylaxis results in death, the most common mechanism is circulatory collapse in cases of venom injections or iatrogenic reactions and respiratory failure in cases of food allergy.22,24
Interestingly, the presence of cutaneous signs appears to be inveresly proportional to the severity of the anaphylactic event.24 In two studies of fatal anaphylaxis it was noted that less than 20% of the subjects had cutaneous findings. 24,26 This stands in contrast to anaphylaxis studied in the office setting where the majority of patients had cutaneous signs.2,3,7,27 This may indicate that mucocutaneous signs take longer to develop than shock and respiratory distress and patients who live long enough to develop such signs are more likely to survive. Data from the pediatric population suggests that cutaneous signs also appear less frequently children than in adults, with about one in every five reported patients not having cutaneous findings. 5 This paucity of cutaneous findings in the most severely affected is likely to contribute to misdiagnosis.
A prior history of asthma appears to be associated with poor outcomes. Almost all reported cases of fatal food related anaphylaxis occurred in individuals with a known history of asthma.16,23,28,29That stated, the severity of previous attacks appears to be an unreliable prognostic tool as the majority of reported case fatalities have a history of only mild allergic reactions.16,23
In a review of 164 cases of all-cause fatal anaphylaxis in Great Britain, about half were noted to be iatrogenic.30 The most common iatrogenic causes reported were anesthetics and antibiotics, and fatal cases had a median time to cardiac arrest of only five minutes. Fatal venom injections had a median time to death of 15 minutes, ranging from 4–120 minutes, but fatal food allergy occurred slower with a median time to death of 30 minutes, ranging from six minutes to six hours. Also of note was that fatal food allergies occurred most commonly in children and younger individuals, whereas fatal iatrogenic anaphylaxis were seen more frequently among older individuals, likely due to higher exposure rates. In cases where death occurred several hours after the onset of symptoms, subjects commonly presented with mild symptoms, which then rapidly progressed during their period of observation. One case series noted that four patients had developed fatal circulatory collapse within minutes of being placed in an upright position and recommended keeping these patients layingflat31
It is recommended that all individuals who have suffered an acute allergic reaction from exposures that might be encountered in nonmedical settings should receive instructions on how to avoid the precipitating allergen if it is known. Before discharge from the ED, patients should also be given a prescription for self-injectable epinephrine for use if anaphylaxis develops. Patients are also recommended to follow up with an allergist and should be given information on how to learn more about allergy from websites such as www.foodallergy.org.
Despite the widespread use of self-injectable epinephrine, it must be stressed to patients that they must still avoid the offending allergen and that the epinephrine can not be relied on as a rescue medication. Auto-injectors have been associated with failures due to technical issues such as incorrect technique of administration or failure of the correctly administered dose to prevent fatality.16,30,32 Similiarly, when epinephrine has been given by health care providers, errors regarding appropriate dosaging and concentration of epinephrine used have been associated with poor patient outcomes. The use of pre-filled injectors by health care providers may avoid such errors.31,33
MARKERS OF ANAPHYLAXIS – TRYPTASE
A detailed clinical history and physical exam may not correctly identify all cases of anaphylaxis. Using laboratory markers as diagnostic adjuncts has been proposed and markers such as prostaglandin D2, carpoxypeptidase, CD63, interleukin 4 and 6, CRP and tryptase have been evaluated for this purpose.34–36 Histamine released by degranulation of mast cells can be measured within five to ten minutes but only remains elevated for 30–60 min and therefore has a very limited value. To date, the marker most widely recommended is serum tryptase.
Tryptase is a neutral protease present in the secretory granules of all mast cells and to a lesser extent in basophiles.37 It is found in two isomers: α-tryptase, which is primarily elevated in those with mastocytosis; and β-tryptase, which is more commonly elevated in anaphylaxis.38 The most widely available assay measures total serum tryptase. One milliliter of serum is required for the assay and must be processed within three days if refrigerated.39 The peak level of serum tryptase in anaphylaxis is one to two hours after the precipitating event and the serum half-life is approximately two hours.40 The ideal time to measure serum tryptase is considered within three hours but significant elevations can be found up to six hours or longer.41–43
In a study on anaphylaxis in children during a food challenge, an elevated tryptase had a sensitivity of 89% and specificity of 88%.44 In a review of 18 patients with confirmed anesthesia anaphylaxis, 12 had elevated serum tryptase levels.42 In the context of an acute event, an elevated tryptase level supports the diagnosis of anaphylaxis. Greater diagnostic accuracy however, appears to be associated with changes from a baseline level. In cases of experimental anaphylaxis in an antivenom trial a single serum tryptase value had a sensitivity of 36% and specificity of 93%, whereas a difference from baseline or delta tryptase level of 2.0 mcg/L had a sensitivity of 73% and specificity of 98%.45 This suggests that it may be useful to obtain a baseline tryptase value during a follow-up visit with the allergist.
USE OF TRYPTASE IN THE EMERGENCY DEPARTMENT
As discussed above, making the correct diagnosis in atypical cases can be challenging for the ED physician and using serum tryptase as a diagnostic adjunct should be considered. Current practice guidelines from the American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology recommend that measuring serum tryptase may be helpful to confirm a diagnosis of anaphylaxis or rule out other causes. The guidelines note that proper timing when obtaining blood for tryptase measurement is essential and should be performed within six hours.46 The United Kingdom Resuscitation Council guidelines on anaphylaxis also recommend using serum tryptase during the acute attack to confirm the diagnosis47
Only two studies have been identified where tryptase was measured specifically in ED patients with acute reactions. A study of patients with confirmed anaphylaxis where serum tryptase was measured within six hours from onset of symptoms and compared to baseline tryptase levels one month later revealed a sensitivity of 94% and specificity 92%.48 In another study where tryptase was measured in 96 ED patients with anaphylaxis, beta-tryptase was detectable in just 23 patients and only 13 had elevated total tryptase levels. These results should be interpreted with caution as the study included patients who presented greater than 12 hours from the onset of symptoms. Applying current guidelines, only a minority of subjects met criteria for anaphylaxis.49
Currently, in most centers, serum tryptase levels are not available as a STAT study which may be a disincentive to those in the acute care setting. That stated, serum tryptase levels can help identify the cause of an atypical acute reaction of unknown etiology and is recommended as a diagnostic adjunct in cases of doubt. The significance of an individual patient’s elevated value should be determined by an allergist and ideally compared to a baseline value at a follow up visit. The patient’s ED visit is however the most opportune time to draw blood for tryptase analysis, and this may provide the follow-up physician with useful information.
CONCLUSION
Reviewing current data on anaphylaxis, it is evident that the definition and standardization of this diagnosis has been variable. Maintaining a high index of suspicion in patients presenting with atypical signs and symptoms can improve diagnostic accuracy and avoid poor outcomes due to misdiagnosis. Emergency physicians should be familiar with the currently recommended diagnostic criteria and the option to measure serum tryptase levels within the first six hours of presentation as a diagnostic adjunct in atypical cases.
Footnotes
Supervising Section Editor: Gene Hern Jr., MD
Submission history: Submitted July 29, 2009; Revision Received October 20, 2009; Accepted December 29, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Charles S Graffeo MD, Department of Emergency Medicine, Eastern Virginia Medical School, Raleigh Building, Room 304, 600 Gresham Drive, Norfolk, VA 23507
Email: graffecs@evms.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. Ann Emerg Med. 2006;47:373–80. [PubMed]
2. Bohlke K, Davis RL, DeStefano F, et al. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. J Allergy Clin Immunol. 2004;113:536–42. [PubMed]
3. Yocum MW, Butterfield JH, Klein JS, et al. Epidemiology of anaphylaxis in Olmsted County: a population-based study. J Allergy Clin Immunol. 1999;104:452–6. [PubMed]
4. Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: A report from the Rochester Epidemiology Project. J Allergy Clin Immunol.2008;122:1161–5. [PMC free article] [PubMed]
5. Braganza SC, Acworth JP, Mckinnon DRL, et al. Pediatric emergency department anaphylaxis: different patterns from adults. Arch Dis Child. 2006;91:159–163. [PMC free article] [PubMed]
6. Klein JS, Yocum MW. Underreporting of anaphylaxis in a community emergency room. J Allerg Clin Immun. 1995;95:637–9.
7. Webb LM, Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol.2006;97:39–43. [PubMed]
8. Kane KE, Cone DC. Anaphylaxis in the prehospital setting. J Emerg Med. 2004;27(4):371–7.[PubMed]
9. Clark S, Bock SA, Gaeta TJ, et al. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004;113:347–52. [PubMed]
10. Pumphrey RSH. Further fatal allergic reactions to food in the United Kingdom, 1999–2006. J Allerg Clin Immunol. 119;(4):1016–18.
11. Nguyen A, Gern J. Food allergy masquerading as foreign body aspiration. J Allergy Clin Immunol. 2000;105(suppl):143. [PubMed]
12. Harukuni I, Ishizawa Y, Nishikawa T, et al. Anaphylactic shock with ventricular fibrillation induced by chlorhexidine. Masui – Japanese J of Anesthesiology. 1992;41(3):455–9.
13. Engrav MB, Zimmerman M. Electrocardiographic changes associated with anaphylaxis in a patient with normal coronary arteries. West J Med. 1994;161:602–4. [PMC free article] [PubMed]
14. Brown SGA, Blackman KE, Stenlake V, et al. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J. 2004;21:149–54.[PMC free article] [PubMed]
15. Ginsburg R, Bristow MR, Kantrowitz N, et al. Histamine provocation of clinical coronary artery spasm: implications concerning pathogenesis of variant angina pectoris. Am Heart J. 1981;102:819–22. [PubMed]
16. Cabaton J, Rondelet B, Gergele L, et al. Takotsubo syndrome after anaphylaxis caused by succinylcholine during general anaesthesia. Annales Francaises d Anesthesie et de Reanimation.2008;27(10):854–7. [PubMed]
17. Attenhofer C, Rudolf S, Salomon F, et al. Ventricular fibrillation in a patient with exercise-induced anaphylaxis, normal coronary arteries and a positive ergonovine test. Chest.1994;105:620–2. [PubMed]
18. Pumphrey RSH. Further fatal allergic reactions to food in the United Kingdom, 1999–2006. J Allerg Clin Immunol. 119;4:1016–8.
19. Nugent JS, Nugent AL, Whisman BA, et al. Levothyroxine anaphylaxis? Vocal cord dysfunction mimicking an anaphylactic drug reaction. Ann Allergy Asthma Immunol. 2003;91(4):337–41.[PubMed]
20. McFadden ER, Zawadski DK. Vocal cord dysfunction masquerading as exercise induced asthma.Am J Respir Crit Care Med. 1996;153:942–7. [PubMed]
21. Nugent JS, Napoli DC. Immunotherapy triggering acute VCD. Allergy. 2002;57(11):1089–90.[PubMed]
22. Soreide E, Buxrud T, Harboe S. Severe anaphylactic reactions outside hospital: etiology, symptoms and treatment. Acta Anaestesiol Scand. 1988;32:339–42.
23. Pumphrey RS. Fatal anaphylaxis in the UK, 1992–2001. Novartis Found Symp. 2004;257:116–28. [PubMed]
24. Liew WK, Williamson E, Tang MLK. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123:434–42. [PubMed]
25. Low I, Stables S. Anaphylactic deaths in Auckland, New Zealand: a review of coronial autopsies from 1985 to 2005. Pathology. 2006;38(4):328–32. [PubMed]
26. Simon MR, Mulla ZD. A population-based epidemiologic analysis of deaths from anaphylaxis in Florida. Allergy. 2008;63:1077–83. [PubMed]
27. Greenberger PA, Rotskoff BD, Lifschultz B. Fatal anaphylaxis: postmortem findings and associated comorbid diseases. Ann Allergy Asthma Immunol. 2007;98:252–7. [PubMed]
28. Brown A, McKinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–6. [PubMed]
29. Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. [PubMed]
30. Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allerg Clin Immunol. 2007;119(4):1016–18.
31. Pumphrey RSH. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Experim Allergy. 2000;30:1144–50.
32. Pumphrey RSH. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol. 2004;4:285–90. [PubMed]
33. Macdougall C, Cant A, Colver A. How dangerous is food allergy in childhood? The incidence of severe and fatal allergic reactions across the UK and Ireland. Arch Dis Child. 2002;86(4):236–9.[PMC free article] [PubMed]
34. Lin RY, Trivino MR, Curry A, et al. Interleukin 6 and C-reactive protein levels in patients with acute allergic reactions: an emergency department-based study. Ann Allergy Asthma Immunol.2001;87:412–6. [PubMed]
35. Sainte-Laudy J, Cado S. Comparison of the levels of histamine, tryptase, and interleukin-6 for the investigation of anaphylactoid drug reactions. Allergie et Immunologie. 1998;30(7):209–11.[PubMed]
36. Erger RA, Sahl B, Casale TB. Human lung anaphylaxis results in rapid release of interleukin-4. Ann Allergy Asthma Immunol. 1997;78(6):566–8. [PubMed]
37. Castells MC, Irani AM, Schwartz LB. Evaluation of human peripheral blood leukocytes for mast cell tryptase. J. Immunol. 1987;138:2184–9. [PubMed]
38. Schwartz LB, Sakai K, Bradford TR, et al. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J. Clin. Invest. 1995;96:2702–10. [PMC free article] [PubMed]
39. Tryptase: ARUP Laboratory Tests. ARUP Laboratories: A National Reference Laboratory.Retrieved from http://www.aruplab.com/guides/ug/tests/0099173.jsp.
40. Schwartz LB, Yueninger JW, Miller J, et al. Time course of appearance and disappearance of human mast cell tryptasein the circulation after anaphylaxis. J Clin Invest. 1989;83:1551–5.[PMC free article] [PubMed]
41. Fisher MM, Baldo BA. Mast cell tryptase in anaesthetic anaphylactoid reactions. Br J Anaesth.1998;80:26–9. [PubMed]
42. Dybendal T, Guttormes AB, Elsayed S, et al. Screening for mast cell tryptase and serum IgE antibodies in 18 patients with anaphylactic shock during general anaesthesia. Acta Anaesthesiol Scand. 2003;47:1211–8. [PubMed]
43. Shanmugam G, Schwartz LB, Khan DA. Prolonged elevation of serum tryptase in idiopathic anaphylaxis. J Allergy Clin Immunol. 2006;117(4):950–1. [PubMed]
44. Narita M, Nomura I, Aota A, et al. Usefulness of Serum Tryptase in the Diagnosis of Anaphylaxis by Food Allergy in Childhood. J Allergy Clin Immunol. 2006;117(2):s307.
45. Brown SGA, Blackman KE, Heddle RJ. Can serum mast cell tryptase help diagnose anaphylaxis?Emerg Med Australasia. 2004;16:120–4.
46. Joint Task Force on Practice Parameters. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;3:S483–523.
47. Emergency treatment of anaphylactic reactions guidelines for healthcare providers. Resucitation Council UK. Jan, 2008. Retrieved from http://www.resus.org.uk/pages/reaction.pdf.
48. Enrique E, Garcia-Ortega P, Sotorra O, et al. Usefulness of UniCAP-tryptase fluoroimmunoassay in the diagnosis of anaphylaxis. Allergy. 1999;54:602–6. [PubMed]
49. Lin RY, Schwartz LB, Curry A, et al. Histamine and tryptase levels in patients with acute allergic reactions: an emergency department based study. J Allergy Clin Immunol. 2000;106:65–71.[PubMed]


