| Author | Affiliation |
|---|---|
| Lorenzo Paladino, MD | State University of New York, Downstate Medical Center, Brooklyn, NY |
| Ramanand A. Subramanian, PhD | State University of New York, Downstate Medical Center, Brooklyn, NY |
| Elisabeth Bonilla, BS | Brooklyn College, Brooklyn, NY |
| Richard H. Sinert, DO | State University of New York, Downstate Medical Center, Brooklyn, NY |
ABSTRACT
Introduction:
To test the diagnostic use of the triage white blood cell (WBC) count in differentiating major from minor injuries.
Methods:
We conducted a retrospective study of a prospectively collected database of trauma patients 13 years of age or older at a Level I trauma center from January 2005 through December 2008. We excluded all patients with obvious life-threatening injuries requiring immediate surgery, isolated head trauma, transferred from another institution or dead on arrival. We recorded age, sex, injury mechanism, vital signs, WBC, base deficit (BD), lactate (LAC) and calculated injury severity scores (ISS). Major injury was defined as either a change in hematocrit >10 points or blood transfused within 24 hours, or ISS >15.
Results:
805 patients were included in the study with an average age of 38.6 years (Range 13–95 yrs) years. 75.3% of patients were male, 45.6% had blunt and 34.4% had penetrating trauma. For vital signs, blood pressure was not significantly different between major and minor injury patients. Major compared to minor injury patients had a statistically but not clinically significant higher heart rate. Major injury patients had significantly (p < 0.0001) higher WBC count (10.53 K/μl, 95% CI: 9.7–11.3) compared to patients with minor injuries (8.92 K/μl, 95% CI: 8.7–9.2), but both were in the normal range. Patients with major compared to minor injury had significantly (p < 0.0001) higher BD (−3.1 versus −0.027 mmol/L) and higher LAC (3.9 versus 2.48 mmol/L). Areas under the curve for WBC count (0.60, 95% CI: 0.54–0.66) are similar to BD (0.69, 95% CI: 0.63–0.74) and LAC (0.66, 95% CI: 0.60–0.71).
Conclusion:
WBC count is not a useful addition as a diagnostic indicator of major trauma in our study population.
INTRODUCTION
Emergency physicians are continually searching for early markers that efficiently differentiate trauma patients with major versus minor injury. Historically Advanced Trauma Life Support taught us the value of injury mechanism, physical exam and vital signs in identifying trauma patients who require emergent workups for suspected life-threatening injuries.1 While hypotension and tachycardia are specific for major injuries, normal vital signs are not sufficiently sensitive to reliably exclude bowel, vascular or solid organ injuries.2–4 Lipsky5 showed that a significant proportion of trauma patients who eventually die had normal vital signs in the emergency department (ED). This has led investigators to manipulate heart rate and blood pressure creating the Shock Index, which has shown only limited success over traditional vital signs in rapidly ruling out significant injuries.6–8
Lactate (LAC) and Base Deficit (BD) both measure the gap between oxygen demand and delivery and are successful markers in hypovolemic shock for predicting trauma mortality and transfusion requirements.9–12 While the addition of BD and LAC measurements in trauma patients with normal vital signs improves the detection of major injury, many patients could still be missed.13
Major injury is associated with a stress-induced neurohumoral response stimulating the secretion of epinephrine and cortisol.14–15 This release of stress hormones after trauma is evidenced by hyperglycemia, which showed a comparable performance to BD and LAC in predicting mortality.16–19
In normal healthy subjects these same stress-induced hormones, epinephrine and cortisol, produce leukocytosis from both bone marrow and splenic sources.20,21,22 It has been hypothesized that elevation of a trauma patient’s white blood cell (WBC) count may be a surrogate marker of neurohumoral activation and be valuable in identifying patients with major injuries. Studies in blunt trauma patients have shown higher WBC counts in their more severely injured patients.23–26
Rovlias et al27 in their study of head trauma patients in the neurosurgical intensive care unit (ICU) showed WBC count was significantly higher in patients with severe head injury compared to those with minor or moderate injury. We hypothesized that the WBC count would be successful in differentiating major from minor injuries in a heterogeneous cohort of blunt and penetrating trauma patients. We tested the diagnostic performance of the initial WBC count in differentiating major from minor injury in trauma patients, specifically excluding those with isolated head trauma.
METHODS
Study Design
This was a retrospective study of a prospectively collected database of trauma patients used to evaluate the utility of initial WBC count, BD and LAC to detect major injury. The local institutional review board (IRB) of State University of New York Downstate Medical Center and Kings County Hospital approved this study. Requirement for informed consent was waived by the IRB.
Study Setting & Population
This study was conducted at Kings County Hospital, a Level 1 trauma center in Brooklyn, New York, which receives 135,000 annual ED visits and has approximately 1,000 major trauma resuscitations a year. Trauma patients 13 years of age or older with significant mechanisms defined by our trauma team activation protocol of blunt or penetrating trauma had blood tests performed as part of their diagnostic evaluation. These trauma patients were enrolled from January 2005 to December 2008. Thirteen years old is the cut-off age for adult trauma patients in our institution. Patients excluded from our study were those with obvious injuries requiring immediate surgery, patients transferred from other institutions or those dead on arrival. We excluded these patients because an initial triage screening test is neither needed nor helpful to determine the course of action. The diagnosis and disposition of these patients are either already determined, as in the case of transfers, or obvious for patients in need of emergent surgery or dead on arrival. Patients who went immediately to the operating room included trans-abdominal gunshot wounds or blunt or penetrating torso injuries that were hemodynamically unstable. The decision was made to go for emergent surgery by the operating surgeon in conjunction with the ED attending. Biomarkers are obviated in these patients who need definitive surgical treatment by mechanism and clinical grounds alone.
Finally, we excluded all patients with a history of isolated head trauma as this injury pattern has already been shown to increase WBC count.27
Study Protocol
The decision to begin a trauma workup in the ED and thus make a patient eligible for this study was based on the trauma team activation protocol. The criteria for trauma team activation defined by our institution are as follows: all penetrating injuries of the trunk including groin, buttocks and neck; all penetrating injuries of the extremities in proximity to major vessels; all blunt trauma with accompanying hypotension; all patients with multiple trauma resulting in pelvic fractures or two or more long bone fractures; all patients with at least one long bone or pelvic fracture associated with a thoracic or head injury; all patients who have fallen two or more stories; all pedestrians who have sustained significant injuries as a result of being struck by a moving vehicle; all patients with central nervous system injury with a history of one of the following: loss of consciousness, posturing, lateralizing signs, open cranial injury, or paralysis; all head injury patients with significant symptoms such as: severe headache, nausea and vomiting, dizziness, or amnesia; and all patients with a trauma score of 13 or less. Patients were enrolled by convenience sampling, and treating physicians were not blinded to vital signs or results of WBC, BD, and LAC testing. Patient workup and treatment were not specified in this study protocol.
Measurements
Academic associates only collected data from patients identified by the trauma team activation protocol. Academic associates are medical or undergraduate students who are trained data abstractors. They are certified to assist our department in data collection during their research elective, only after passing the “Protection of Human Subjects” course offered by the IRB. Data collectors were not involved in patient care.
Demographic data, vital signs, including systolic blood pressure (ED-SBP), diastolic blood pressure (ED-DBP) and heart rate (ED-HR), as well as mechanism of injury and physical findings were recorded for all patients. Following resuscitation and trauma workup, all information regarding the recorded injuries was collected, including the results of imaging studies, invasive procedures (diagnostic peritoneal lavage, angiography, chest tube insertion, etc.) and findings of operative diagnostic or therapeutic procedures. Serial hematocrit and the number of units of blood transfused in the patients’ first 24 hours of hospitalization were also recorded. We calculated injury severity scores (ISS) for all patients according to the 1990 revision of the Abbreviated Injury Scale.
As part of our routine trauma evaluation, arterial blood gases (Radiometer ABL 725, Copenhagen, Denmark) were drawn concurrently with the recording of triage vital signs during the initial assessment. Blood testing for all trauma patients included an initial complete blood count, arterial BD, and LAC levels.
Data Analysis
Predictor variables were WBC count and metabolic parameters (BD and LAC). Normal levels of BD were defined as greater than −2.0 mMol/L, and normal LAC were defined as less than 2.2 mMol/L, both based on our hospital’s normal values. By definition, these levels of BD and LAC are outside the 95% confidence interval for their normal values in our population. We chose these values to maximize the sensitivity of these metabolic parameters.
Outcome variables included minor or major injury. Patients with major injury were defined as those with any of the following: patients receiving a blood transfusion within the first 24 hours, having a decrease in hematocrit of greater than ten percentage points in the first 24 hours, or any trauma patient with an ISS > 15. An ISS ≤ 15 has previously been used in the literature to define minor injury.23 Blood transfusion requirement and decrease in hematocrit have been shown to be accurate indicators of major injury in previous studies. In earlier studies done at our center, trauma patients without these criteria (minor injury) had a mortality rate of less than 1%.29–31
Data were reported as means ± standard deviations, or cell counts and percentages with 95% confidence intervals. Group comparisons were analyzed by student’s t-tests or chi-square; where appropriate, all tests were two tailed (SPSS version 15.0, Chicago, Illinois). The alpha value was set at 0.05. Difference between proportions with 95% confidence intervals was determined by using the methods of Newcombe.32 Receiver operator characteristic (ROC) curves were generated for initial WBC count, BD, LAC and vital signs (ED-SBP, ED-DBP, ED-HR) discriminating major from minor injuries in trauma patients, using Analyze-It version 1.73, Analyze-It Software Ltd. United Kingdom.
RESULTS
Between January 2005 to December 2008, we studied 805 trauma patients with a mean age of 38.6 years (Range 13–95 yrs.). 75.3% (n = 606) of patients were male. Mechanisms of injury were blunt, penetrating, falls and other (Table 1).
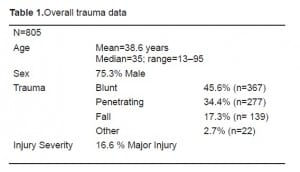
We subdivided study patients into the main outcome variables, minor versus major injury. One hundred thirty-four (16.6 %) patients met criteria for major injury, and 671 (83.4 %) were classified as minor injury.
Table 2 compares the demographics, mechanisms of injury, vital signs and metabolic parameters between minor and major injury patients. There were no differences in age between the major and minor injury groups;75.4% (n = 505) of patients with major injury and 75.3 % (n = 101) of patients with minor injury were males. There was no difference in ED-SBP and ED-DBP between the major and minor injury groups. Only ED-HR had a statistical (p<0.0001) but not a clinical difference between the two groups (mean difference of 8.36 mmHg, 95 % CI: 4.8–11.9). BD was significantly (p<0.0001) larger (mean difference of 3.06 mmol/L, 95% CI, 2.31 to 3.82 mmol/L) in the major compared to minor injury patients. Major injury patients also had a significantly (p<0.0001) higher LAC by a mean difference of 1.39 mmol/L (95% CI, 0.85 mmol/L to 1.94 mmol/L) compared to the minor injury group.
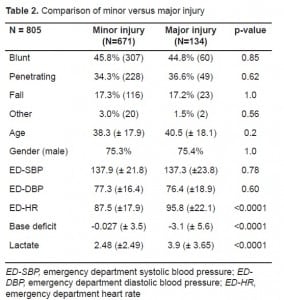
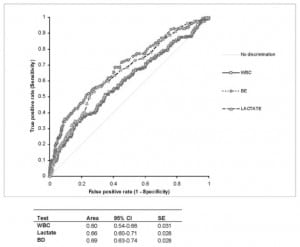
Patients with a major injury (10.53 K/μl, 95% CI: 9.7 to 11.3) had a significantly (p<0.0001) higher WBC count compared to those with minor injury (8.92 K/μl, 95% CI: 8.7 to 9.2). However, the average WBC count in both major and minor injuries was within normal range (4.5 to 10.9 K/μl). The areas under the curve for ED-SBP, ED-DBP and ED-HR were 0.51, 0.49, and 0.61 respectively (ROC curves not shown). Figure 1 compares the ROC curves for WBC counts, BD and LAC in differentiating major from minor injury in trauma patients. The area under the curve for WBC counts (0.60, 95% CI: 0.54 to 0.66) is not significantly different than LAC (0.66, 95% CI: 0.60 to 0.71) or BD (0.69, 95% CI: 0.63 to 0.74). The area under the curve for WBC in only patients with normal vital signs was 0.55 (95 % CI: 0.46–0.64).
DISCUSSION
We studied the use of the WBC count as an initial triage screening test in the ED to distinguish trauma patients with major versus minor injury. We found that even though the WBC count in patients with major (10.53 K/μl) compared to minor (8.92 K/μl) injury was significantly higher, the average WBC counts in both cases were in the normal range. The area under the ROC curve for WBC counts in all patients (0.60) and those with only normal vital signs (0.55) was a poor discriminator of major versus minor injuries.
The literature has shown variable conclusions about the use of the WBC count for screening trauma patients. Several studies have described a significantly higher WBC counts in their most severely injured trauma patients. Morell et al.23 in their study of 156 blunt trauma patients found a statistically significant elevation in WBC counts in patients with an elevated ISS (p<0.002) and longer ICU stays (p<0.006), but failed to demonstrate a linear relationship between WBC counts and these outcome variables. Santucci and colleagues24 retrospectively studied 279 blunt trauma patients and found a statistically higher (p<0.001) mean WBC count in patients with a significant injury compared to those without, but also found a poor correlation (r = 0.369) with ISS. Holmes et al.25 performed a retrospective study of 1040 children under 15 years of age with blunt trauma admitted to their Level 1 trauma center. They concluded that moderate risk patients with intra-abdominal injuries (IAI) (n = 22) had higher mean WBC counts compared to those without IAI. However, none of these three research groups were able to identify any clinically useful WBC cutoffs to reliably exclude major injury.
Chang and colleagues26 prospectively studied 882 blunt and penetrating trauma patients to determine the association of WBC count with demographics, mechanism and severity of injury, outcomes, and the need for therapeutic interventions. They found that variations in WBC count were associated with race and injury severity, but they were not useful in predicting the need for volume resuscitation, transfusion or surgery. However, in patients with a Glasgow Coma Scale ≤ 8, the mean WBC count was more than the upper limit of normal (12,000) suggesting that head injury maybe a confounding variable that leads to an elevation in WBC count. Because of head injury being a possible confounder in the above poly-trauma studies we specifically excluded isolated head trauma patients. The correlation between head trauma severity and elevations in WBC counts is also clearly demonstrated in the study by Rovlias and Kotsou27. In their prospective study of 624 patients with isolated head injury, WBC counts were not only higher in more severe head injuries but also correlated with degree of severity and neurologic outcomes.
LIMITATIONS
Our study population excludes patients who were unstable and/or who met criteria for rapid surgical exploration (e.g., gunshot to abdomen) because arterial blood gas measurements were not taken before their operating room transfer. Patients were enrolled by convenience sample because data abstractors were not present 24 hours a day. Because of this time limit, a significant percentage of trauma patients may have been missed. The above conditions restricted the number of eligible study patients and may explain why only 805 trauma patients were enrolled over the study period in a large Level 1 trauma center. Excluding patients who rapidly went to surgery may have falsely depressed the evidence of stress leukocytosis in our trauma subjects. These limitations may affect the applicability of our results regarding patients who are unstable and in need of emergent surgery, but provide valuable data for patients with occult injuries. We used ROC curve analysis and not a multivariate technique to look for a clinically significant cutoff for WBC.
CONCLUSION
Our findings in a heterogeneous blunt and penetrating trauma cohort (excluding isolated head injury) support the findings of previous studies of blunt cohorts: although there is a statistically significant higher WBC count in patients with major injuries, no clinical cutoffs or use are identified. WBC count was not a useful addition as a diagnostic indicator of major trauma in our study population.
Footnotes
Supervising Section Editor: Christopher A. Kahn, MD, MPH
Submission history: Submitted December 10, 2009; Revision Received February 16, 2010; Accepted April 21, 2010
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Ramanand A. Subramanian PhD, SUNY Downstate, Department of Emergency Medicine, Box 1228, 450 Clarkson Ave, Brooklyn, NY 11203
Email: Arun.Subramanian@downstate.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Kortbeek JB. Advanced trauma life support, 8th edition, the evidence for change. J Trauma.2008;64(6):1638–50. [PubMed]
2. Brown CV, Velmahos GC, Neville AL, et al. Hemodynamically ”stable” patients with peritonitis after penetrating abdominal trauma: identifying those who are bleeding. Arch Surg. 2005;140:767–72. [PubMed]
3. Adams SL, Greene JS. Absence of a tachycardic response to intraperitoneal hemorrhage. J Emerg Med. 1986;4:383–9. [PubMed]
4. Thompson D, Adams SL, Barrett J. Relative bradycardia in patients with isolated penetrating abdominal trauma and isolated extremity trauma. Ann Emerg Med. 1990;19:268–75. [PubMed]
5. Lipsky AM, Gausche-Hill M, Henneman PL, et al. Prehospital hypotension is a predictor of the need for an emergent, therapeutic operation in trauma patients with normal systolic blood pressure in the emergency department. J Trauma. 2006;61:1228–33. [PubMed]
6. King RW, Plewa MC, Buderer NM, et al. Shock index as a marker for significant injury in trauma patients. Acad Emerg Med. 1996;3:1041–5. [PubMed]
7. Zarzaur BL, Croce MA, Fischer PE, et al. New vitals after injury: shock index for the young and age x shock index for the old. J Surg Res. 2008;147:229–36. [PubMed]
8. Cancio LC, Wade CE, West SA, et al. Prediction of mortality and of the need for massive transfusion in casualties arriving at combat support hospitals in Iraq. J Trauma. 2008;64:S51–5. discussion S5–6. [PubMed]
9. Callaway DW, Shapiro NI, Donnino MW, et al. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma. 2009;66:1040. [PubMed]
10. Husain FA, Martin MJ, Mullenix PS, et al. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185:485–91. [PubMed]
11. Smith I, Kumar P, Molloy S, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001;27:74–83. [PubMed]
12. Davis JW, Parks SN, Kaups KL, et al. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. 1996;41(5):769–74. [PubMed]
13. Paladino L, Sinert R, Wallace D, et al. The utility of base deficit and arterial lactate in differentiating major from minor injury in trauma patients with normal vital signs. Resuscitation.2008;77:363–8. [PubMed]
14. Hetz W, Kamp HD, Zimmermann U, et al. Stress hormones in accident patients studied before admission to hospital. J Accid Emerg Med. 1996;13:243–7. [PMC free article] [PubMed]
15. Greisen J, Juhl CB, Grofte T, et al. Acute pain induces insulin resistance in humans.Anesthesiology. 2001;95:578–84. [PubMed]
16. McNamara JJ, Molot M, Stremple JF, et al. Hyperglycemic response to trauma in combat casualties. J Trauma. 1971;11:337–9. [PubMed]
17. Lange MP, Dahn MS, Jacobs LA. The significance of hyperglycemia after injury. Heart Lung.1985;14:470–2. [PubMed]
18. Bochicchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–4. [PubMed]
19. Wahl WL, Taddonio M, Maggio PM, et al. Mean glucose values predict trauma patient mortality. J Trauma. 2008;65:42–7. discussion 7–8. [PubMed]
20. Toft P, Helbo-Hansen HS, Tonnesen E, et al. Redistribution of granulocytes during adrenaline infusion and following administration of cortisol in healthy volunteers. Acta Anaesthesiol Scand.1994;38:254–8. [PubMed]
21. Landmann R, Durig M, Gudat F, et al. Beta-adrenergic regulation of the blood lymphocyte phenotype distribution in normal subjects and splenectomized patients. Adv Exp Med Biol.1985;186:1051–62. [PubMed]
22. Bessey PQ, Watters JM, Aoki TT, et al. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984;200:264–81. [PMC free article] [PubMed]
23. Morell V, Lundgren E, Gillott A. Predicting severity of trauma by admission white blood cell count, serum potassium level, and arterial pH. South Med J. 1993;86(6):658–9. [PubMed]
24. Santucci CA, Purcell TB, Mejia C. Leukocytosis as a predictor of severe injury in blunt trauma.West J Emerg Med. 2008;9:81–5. [PMC free article] [PubMed]
25. Holmes JF, Sokolove PE, Land C, et al. Identification of intraabdominal injuries in children hospitalized following blunt torso trauma. Acad Emerg Med. 1999;6:799–806. [PubMed]
26. Chang DC, Cornwell EE, III, Phillips J, et al. Early leukocytosis in trauma patients: what difference does it make? Curr Surg. 2003;60:632–5. [PubMed]
27. Rovlias A, Kotsou S. The blood leukocyte count and its prognostic significance in severe head injury. Surg Neurol. 2001;55:190–6. [PubMed]
28. Mattox KL, Feliciano DV, Moore EE. Trauma. 4th ed. McGraw-Hill; 1999. p. 87.
29. Malone DL, Dunne J, Tracy JK, et al. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54(5):898–905. [PubMed]
30. Paradis NA, Balter S, Davison CM, et al. Hematocrit as a predictor of significant injury after penetrating trauma. Am J Emerg Med. 1997;15(3):224–8. [PubMed]
31. Zehtabchi S, Sinert R, Baron BJ, et al. Does ethanol explain the acidosis commonly seen in ethanol-intoxicated patients? Clin Toxicol (Phila) 2005;43:161–6. [PubMed]
32. Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–90. [PubMed]


