| Author | Affiliation |
|---|---|
| Getaw worku Hassen, MD, PhD | New York Medical College, Metropolitan Hospital Center, Department of Emergency Medicine, Westchester County, New York |
| Mona Milkha Singh, MD | New York Medical College, Metropolitan Hospital Center, Department of Emergency Medicine, Westchester County, New York |
| Hossein Kalantari, MD | New York Medical College, Metropolitan Hospital Center, Department of Emergency Medicine, Westchester County, New York |
| Selamawit Yemane-Merriwether, MD | New York Medical College, Metropolitan Hospital Center, Department of Emergency Medicine, Westchester County, New York |
| Steven Ferrante, PA | New York Medical College, Metropolitan Hospital Center, Department of Emergency Medicine, Westchester County, New York |
| Ronald Shaw, MD | New York Medical College, Metropolitan Hospital Center, Department of Emergency Medicine, Westchester County, New York |
ABSTRACT
Pulmonary embolism (PE) is a life-threatening condition that may present as dyspnea, chest pain, cough or hemoptysis, but often occurs without symptoms. It is not typically associated with hiccups. Hiccups are generally self-limiting benign contractions of the diaphragm that may be associated with medications or food but may also be symptomatic of serious disease when persistent. We report 3 cases of PE presenting as persistent hiccups.
INTRODUCTION
Pulmonary embolism (PE) is a potentially lethal condition that can be difficult to diagnose. The incidence in the United States is as high as 1 per 1,000 persons per year, and it is the third commonest cause of death in hospitalized patients.1 Early diagnosis and treatment are crucial in ensuring a good outcome, but symptoms are non-specific and the index of suspicion often low.2–4 As a result the diagnosis is often made post-mortem.5
Hiccup or singultus is the involuntary, spasmodic contraction of the inspiratory muscles, especially the diaphragm. Hiccups are believed to be due to stimulation of the hiccup reflex arc and are generally transient and innocuous. Sometimes they are persistent and symptomatic of organic diseases in the nervous, respiratory, cardiovascular or digestive systems.6–12
The hiccup reflex arc consists of an afferent limb that includes the phrenic and vagus nerves, plus sympathetic fibers from T6–10; a center in the brainstem; and an efferent limb consisting mainly of the phrenic nerve. Irritation of any part of the arc in the head, neck, chest or abdomen can lead to hiccups. The irritant may be inflammation, medication, trauma or even over distension of a viscus.6–8, 12, 13
Hiccups have been associated with medications (steroids, dopamine, azithromycin, cefotetan, benzodiazepines, propofol),CNS disorders (tumors and vascular anomalies, multiple sclerosis and seizures), pulmonary disease (lung cancer), gastric and esophageal disease (GERD, herpetic esophagitis, gastric volvulus) and cardiac conditions (myocardial infarction, pacemaker lead injury).7, 9, 12, 14–17 While many disease entities have been associated with hiccups, we found only one other report of pulmonary embolism presenting as hiccups.28–33
Pulmonary embolism may cause irritation of the afferent or efferent limb in the chest, although the exact mechanism that causes hiccups is unclear.
We report here 3 cases of pulmonary embolism presenting as persistent hiccups.
CASE DESCRIPTION
A search of the medical literature (search for PE and intractable hiccups through Medline, the Cochrane library database and Google search engine) did not reveal any case series of hiccups as a presenting symptom of PE, although a single citation describing this entity was identified in the lay literature and one case report.33–34 In 2 of our 3 patients, recent surgery was a risk factor for PE.
Patient # 1
A 52-year-old African-American male without significant prior medical or surgical history presented to the emergency department (ED) with a 3-day history of hiccups. He had had progressively worsening dyspnea for 4 weeks, a dry cough and pain in the upper right back associated with inspiration and coughing. He had increasing leg edema for 2 weeks and was told he had congestive heart failure at another institution, where he was advised admission, but left against medical advice. The rest of his review of systems (ROS) was unremarkable and he had no risk factors for deep venous thrombosis (DVT), such as family history of clotting disorder or personal history of immobilization, long journeys, surgery or trauma. He had quit occasional smoking many years ago and did not use alcohol or narcotic drugs.
Physical examination revealed a well-built male in mild respiratory distress with a respiratory rate (RR) of 20 breaths/minute, pulse rate (PR) of 108 beats/minute and blood pressure (BP) 113/76 mmHg. His oxygen saturation was 89–91% on room air. His electrocardiogram (EKG) was normal except for sinus tachycardia, and a duplex ultrasound of his lower extremities was negative for DVT. His portable chest radiograph (CXR) was the first hint of abnormality (Figure 1), showing a prominent pulmonary central artery (early Fleishner’s sign, red arrow heads) and a pruned tree /cut off of the pulmonary arteries (Westermark’s sign, black arrows). With the working diagnosis of PE, a computed tomography (CT) of the chest with intravenous (IV) contrast was performed which revealed a massive saddle embolus of the pulmonary artery (Figure 2A, black arrow). Patient was heparinized and transferred to a tertiary center where pulmonary thrombectomy was performed. He made a full recovery with resolution of hiccups and dyspnea. His laboratory examination with complete blood count (CBC), complete metabolic panel (CMP), prothrombin time (PT)/international normalize ration (INR) and partial thromboplastin time (PTT) was essentially normal. Workup for a coagulation disorder failed to reveal a reason for his embolus.
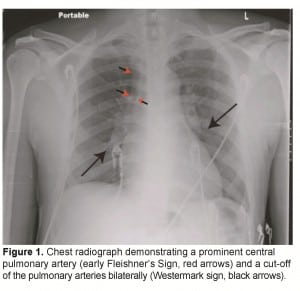
Chest radiograph demonstrating a prominent central pulmonary artery (early Fleishner’s Sign, red arrows) and a cut-off of the pulmonary arteries bilaterally (Westermark sign, black arrows).
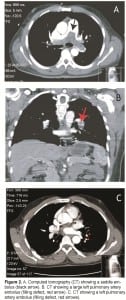
A. Computed tomography (CT) showing a saddle embolus (black arrow). B. CT showing a large left pulmonary artery embolus (filling defect, red arrow). C. CT showing a left pulmonary artery embolus (filling defect, red arrows).
Patient # 2
A 40-year-old Hispanic female came for her follow-up gynecology appointment after undergoing total abdominal hysterectomy for fibroids under general anesthesia 12 days earlier. She complained of severe left lower back pain of 1-day duration and was referred to the ED to rule out renal colic. Her postoperative course was uneventful and she had been ambulating on post-operative day #1 and discharged home shortly after surgery. In the ED she spoke in fragmented sentences due to persistent hiccups. ROS and past medical history were unremarkable
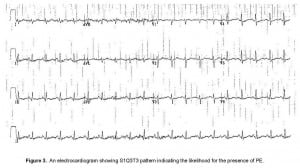
Physical examination revealed a young female with hiccups and mild lower abdominal tenderness, but no signs of rhonchi, rales or crepitations in the chest. She was given dilaudid and morphine for back pain, but her PR was 110–120 beats/minute and BP 112/78 mmHg. Temperature was 100.7° Fahrenheit and RR 20 breaths/minute. Based on the previous case of PE presenting with hiccups, a diagnosis of pulmonary embolism was entertained. CXR was unremarkable, but EKG revealed S1T3Q3 changes (Figure 3). CT chest revealed a large left pulmonary embolus (Figure 2B, red arrow). Bedside duplex ultrasound did not show DVT. Her laboratory examination with CBC, CMP, PT, INR, and PTT was essentially normal. Workup for a coagulation disorder failed to reveal a reason for his embolus. She was started on IV heparin infusion and warfarin and discharged home with resolution of hiccups and back pain.
Patient #3
A 46-year old African-American male presented to the ED with persistent hiccups 2 days after having left inguinal herniorrhaphy under general anesthesia. Surgery was uncomplicated and performed as an ambulatory procedure. He had been ambulating at home, but one day after surgery developed hiccups. His past medical history was unremarkable, but he had undergone right inguinal herniorrhaphy in 2008 without any adverse events or symptoms. He was a nonsmoker and drank no alcohol and denied any other risk factors for DVT.
Physical examination revealed a middle-aged man in no apparent distress except for hiccups. At presentation his temperature was 98° Fahrenheit, PR 81 beats/minute, BP 128/77 mmHg and RR 18 breaths/minute. ECG showed normal sinus rhythm. He was given chlorpromazine, which resolved his hiccups, and he was discharged home. On his way home the hiccups returned and he came back to the ED. A second dose of chlorpromazine failed to resolve the hiccups this time. He also complained of feeling dizzy and his BP was found to be low. A chest CT was ordered and revealed a pulmonary embolus (Figure 2C, red arrow heads). His laboratory examination with CBC, CMP, PT, INR, and PTT was essentially normal. Workup for a coagulation disorder failed to reveal a reason for his embolus.
DISCUSSION
The symptoms and signs of PE are highly variable and non-specific and a high index of suspicion is critical in the diagnosis of this potentially lethal condition. The most common symptoms of PE are dyspnea at rest or on exertion (73%), pleuritic chest pain (44%), and cough (34%) with calf pain (44%) and calf or thigh swelling(41%) indicating DVT preceding PE.35, 36 Patients with such symptoms or signs often need additional testing to confirm or exclude the diagnosis. Hiccups on the other hand are rarely associated with PE. Patients may also be completely asymptomatic, making the diagnosis more challenging. A meta-analysis of 28 studies found that among 5,233 patients who had DVT, 1,665 (32%) had asymptomatic PE, highlighting the importance of a high index of suspicion.37Risk stratification or pretest probability assessment thus becomes paramount in raising awareness of a possible diagnosis of PE.
All patients with chest pain or shortness of breath should have CXR and ECG. In patients with high pretest probability and non-specific symptoms, even if CXR and EKG are normal d-dimer level, lower extremity duplex scan, ventilation-perfusion (VQ) scans and CT angiogram may be needed.35, 37–40
Numerous prediction models and algorithms have been developed for assessment of patients with possible PE, including the Geneva, Kline, Pulmonary Embolism Rule-out Criteria (PERC), Pisa and Wells systems.41–46 Of these the Wells criteria and the PERC rules are the most popular. The different elements included in these scoring systems are clinical history, physical examination, and diagnostic tests, such as arterial blood gas, D-dimer, ECG, and CXR with points ascribed to each element. Based on the total score and the clinical picture, the need for additional testing, such as VQ scans and CT angiograms, is determined by the clinician. Thus, by PERC rules if a patient meets all 8 criteria and falls into a low-risk category the probability of PE is < 2% and further testing may not be required. By Wells’ criteria, a patient with low probability of PE and a negative D-dimer test may be discharged home without more testing.
Zylicz reported a case of intractable hiccups due to PE suggesting that a thrombus in the inferior vena cava (shown on ultrasound) caused PE and then hiccups, but no objective evidence of PE is presented.33 Hiccups resolved with low molecular weight heparin, but recurred when heparin was discontinued. The patient also had underlying non-small cell lung cancer and it is unclear if lung cancer was the source of the hiccups. All of our patients had CT-documented PE and 2 had the risk factor of recent surgery.
Patient # 1 had a low Wells score of 1.5 (HR > 100), making him low risk, but he failed 3 of 8 PERC criteria and the CXR and overall gestalt raised suspicion for PE. Also the Kline decision rule (age/hypoxemia) put him in the unsafe category with a pretest probability of 45.2%. The revised Geneva score placed him in a moderate risk group based on tachycardia alone. Patient # 2 had a score of 3 on Wells criteria (HR > 100; recent surgery) and failed 2 of 8 PERC categories. In patient #3 Wells score was 1.5 (recent surgery), and he failed only 1 of 8 PERC categories.
Different scoring systems put patients in different risk categories highlighting the difficulty in relying only on one scoring system. Intractable hiccups in 2 patients raised our level of suspicion after our experience with patient #1, although both of these patients had recent surgery. CXR in patient #1 and EKG in patient # 2 also helped point us in the right direction. Our series of 3 patients with PE who had hiccups as a presenting symptom highlights the importance of including hiccups in the constellation of symptoms and signs associated with PE. If pretest probability testing is also taken into account, it will help us decide which patients need more advanced imaging and testing to confirm or exclude the diagnosis.
CONCLUSION
Hiccups are common and often idiopathic, but persistent hiccups should be taken seriously. They may be manifestations of immediately life-threatening conditions like myocardial infarction or even pulmonary embolism. Including hiccups in the pantheon of symptoms associated with PE will raise awareness as demonstrated by our small series.
Footnotes
The authors would like to thank Drs. Mariadason and Zehtabchi for reviewing and proofreading the manuscript.
Supervising Section Editor: Rick A. McPheeters, DO
Submission history: Submitted September 13, 2011; Revision received March 23, 2012; Accepted April 2, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.4.6894
Address for Correspondence: Getaw worku Hassen MD, PhD, New York Medical College, Metropolitan Hospital Center, Department of Emergency Medicine, Westchester County, New York
Email: getawh@yahoo.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037–52. [PubMed]
2. Kline JA, Hernandez-Nino J, Jones AE, et al. Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14:592–8. [PubMed]
3. Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247–55. [PubMed]
4. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165–71. [PubMed]
5. Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82:203–5.[PMC free article] [PubMed]
6. Gigot AF, Flynn PD. Treatment of hiccups. J Am Med Assoc. 1952;150:760–4.[PubMed]
7. Lewis JH. Hiccups: causes and cures. J Clin Gastroenterol. 1985;7:539–52. [PubMed]
8. Marai I, Levi Y. [The diverse etiology of hiccups] Harefuah. 2003;142:10–3. 79.[PubMed]
9. Martinez Rey C, Villamil Cajoto I. [Hiccup: review of 24 cases] Rev Med Chil.2007;135:1132–8. [PubMed]
10. Nathan MD, Leshner RT, Keller AP., Jr Intractable hiccups. (singultus) Laryngoscope.1980;90:1612–8. [PubMed]
11. Rousseau P. Hiccups. South Med J. 1995;88:175–81. [PubMed]
12. Souadjian JV, Cain JC. Intractable hiccup. Etiologic factors in 220 cases. Postgrad Med. 1968;43:72–7. [PubMed]
13. Loft LM, Ward RF. Hiccups. A case presentation and etiologic review. Arch Otolaryngol Head Neck Surg. 1992;118:1115–9. [PubMed]
14. Errante D, Bernardi D, Bianco A, et al. Recurrence of exhausting hiccup in a patient treated with chemotherapy for metastatic colon cancer. Gut. 2005;54:1503–4.[PMC free article] [PubMed]
15. Takiguchi Y, Watanabe R, Nagao K, et al. Hiccups as an adverse reaction to cancer chemotherapy. J Natl Cancer Inst. 2002;94:772. [PubMed]
16. Miyaoka H, Kamijima K. Perphenazine-induced hiccups. Pharmacopsychiatry.1999;32:81. [PubMed]
17. Thompson DF, Landry JP. Drug-induced hiccups. Ann Pharmacother. 1997;31:367–9. [PubMed]
18. Arami MA. A case of brainstem cavernous angioma presenting with persistent hiccups. Acta Med Iran. 48:277–8. [PubMed]
19. Dickerman RD, Jaikumar S. The hiccup reflex arc and persistent hiccups with high-dose anabolic steroids: is the brainstem the steroid-responsive locus? Clin Neuropharmacol. 2001;24:62–4. [PubMed]
20. McFarling DA, Susac JO. Hoquet diabolique: intractable hiccups as a manifestation of multiple sclerosis. Neurology. 1979;29:797–801. [PubMed]
21. Nickerson RB, Atchison JW, Van Hoose JD, et al. Hiccups associated with lateral medullary syndrome. A case report. Am J Phys Med Rehabil. 1997;76:144–6. [PubMed]
22. de Hoyos A, Esparza EA, Cervantes-Sodi M. Non-erosive reflux disease manifested exclusively by protracted hiccups. J Neurogastroenterol Motil. 16:424–7.[PMC free article] [PubMed]
23. Hackworth WA, Kimmelshue KN, Stravitz RT. Peritoneal sarcoidosis: a unique cause of ascites and intractable hiccups. Gastroenterol Hepatol (N Y) 2009;5:859–61.[PMC free article] [PubMed]
24. Haas C, Degoutte E, Biclet P, et al. [Intractable hiccup caused by hiatal hernia with esophagitis] Presse Med. 1989;18:634. [PubMed]
25. Kounis NG. Persistent hiccuping in acute myocardial infarction–report of a case. Ir Med J. 1974;67:644–5. [PubMed]
26. Krysiak W, Szabowski S, Stepie M, et al. Hiccups as a myocardial ischemia symptom.Pol Arch Med Wewn. 2008;118:148–51. [PubMed]
27. Celik T, Kose S, Bugan B, et al. Hiccup as a result of late lead perforation: report of two cases and review of the literature. Europace. 2009;11:963–5. [PubMed]
28. Cheng MH, Twu NF, Fuh JL, et al. Intractable hiccups as an unusual presentation of a uterine leiomyoma: a case report. J Reprod Med. 2005;50:954–6. [PubMed]
29. Jones JS, Lloyd T, Cannon L. Persistent hiccups as an unusual manifestation of hyponatremia. J Emerg Med. 1987;5:283–7. [PubMed]
30. Kumar A, Dromerick AW. Intractable hiccups during stroke rehabilitation. Arch Phys Med Rehabil. 1998;79:697–9. [PubMed]
31. Marinella MA. Diagnosis and management of hiccups in the patient with advanced cancer. J Support Oncol. 2009;7:122–7. 130. [PubMed]
32. Payne BR, Tiel RL, Payne MS, et al. Vagus nerve stimulation for chronic intractable hiccups. Case report. J Neurosurg. 2005;102:935–7. [PubMed]
33. Zylicz Z. Intractable hiccups caused by pulmonary embolism. A case report. Adv. Pall. Med. 2009;4:149–152.
34. Cal Shipley. Pulmonary Embolism – a review by Cal Shipley, M.D. Available at:http://www.trialimagestore.com/article_pulmonary_embolism.html. Accessed: 09, 2011.
35. Courtney DM, Kline JA, Kabrhel C, et al. Clinical features from the history and physical examination that predict the presence or absence of pulmonary embolism in symptomatic emergency department patients: results of a prospective, multicenter study. Ann Emerg Med. 55:307–315. [PMC free article] [PubMed]
36. Kabrhel C, McAfee AT, Goldhaber SZ. The contribution of the subjective component of the Canadian Pulmonary Embolism Score to the overall score in emergency department patients. Acad Emerg Med. 2005;12:915–20. [PubMed]
37. Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Radiology. 2007;242:15–21.[PubMed]
38. Daniel KR, Jackson RE, Kline JA. Utility of lower extremity venous ultrasound scanning in the diagnosis and exclusion of pulmonary embolism in outpatients. Ann Emerg Med. 2000;35:547–54. [PubMed]
39. Pini M, Marchini L, Giordano A. Diagnostic strategies in venous thromboembolism.Haematologica. 1999;84:535–40. [PubMed]
40. Stein PD, Hull R, Patel K, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602.[PubMed]
41. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772–80. [PubMed]
42. Kline JA, Nelson RD, Jackson RE, et al. Criteria for the safe use of D-dimer testing in emergency department patients with suspected pulmonary embolism: a multicenter US study. Ann Emerg Med. 2002;39:144–52. [PubMed]
43. Klok FA, Mos IC, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168:2131–6. [PubMed]
44. Miniati M, Monti S, Bottai M. A structured clinical model for predicting the probability of pulmonary embolism. Am J Med. 2003;114:173–9. [PubMed]
45. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–20. [PubMed]
46. Wicki J, Perneger TV, Junod AF, et al. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med. 2001;161:92–7.[PubMed]