| Author | Affiliation |
|---|---|
| Matthew Silver, MD | Kaiser Permanente, San Diego Medical Center, Department of Emergency Medicine, San Diego, California |
ABSTRACT
We describe the case of a 28-year-old-male with no significant medical history who presented with right-sided hemiparesis, bruits over the carotid and subclavian arteries and an elevated erythrocyte sedimentation rate. Imaging studies revealed a middle cerebral artery thrombus and inflammatory changes of the carotid and subclavian arteries and aorta. The diagnosis of Takayasu’s arteritis was made and the patient was started on steroids and immunomodulators with good clinical response.
INTRODUCTION
While stroke is the third most common cause of death in the United States (US), affecting more than 2.4 million patients per year, only 3% of strokes occur in patients under the age of 40.1–3 Atherosclerotic and embolic disease are common causes of ischemic stroke in both young and old patients. In the young however, a wider array of systemic and vascular diseases must be given consideration. Systemic inflammatory or autoimmune diseases, hypercoagulable states and vascular diseases such as dissection are responsible for about 20% of cases, while no certain cause is found in about one-third of young stroke victims.3 Non-cerebrovascular entities must also be given consideration in the young patient presenting with symptoms suggestive of stroke. Todd’s paralysis, complex migraines and conversion disorders are more likely to afflict the young and can manifest with a similar clinical picture.
CASE REPORT
A 28-year-old male with no significant past medical history presented to a community emergency department with acute onset of a dense right hemiplegia and aphasia. While at work the patient had a witnessed collapse to the ground. 911 was called.
A family member reported that the patient had been complaining of headaches and, back and shoulder pain for two weeks. No other systemic symptoms predated the patient’s presentation. The patient had a history of mild intermittent asthma and used an albuterol metered dose inhalers as needed. There was no known family history of cardiac, cerebrovascular or autoimmune disease.
On presentation, the patient was acutely ill appearing. There were no visible signs of trauma. He had a blood pressure of 111/62 mmHg in the right upper extremity and 173/105 mmHg in the left upper extremity. His heart rate was 115 beats-per-minute and his respiratory rate was 14 breaths per minute with an oxygen saturation of 98% on 3L nasal cannula. He had loud bruits heard over the carotid and subclavian arteries as well as the abdominal aorta. There was a 3/6 systolic ejection murmur noted over the entire precordium. He was non-verbal, not following commands, and did not open his eyes spontaneously. He had extensor posturing to noxious stimuli on the right side.
The patient was intubated for airway protection and taken emergently for a non-contrast brain computed tomography (CT) that revealed a dense middle cerebral artery (MCA) sign on the left corresponding to a thrombus without acute hemorrhage, infarct or mass effect (Figure 1). The patient was administered alteplase within a 4.5 hour window without any immediate change in his clinical status.
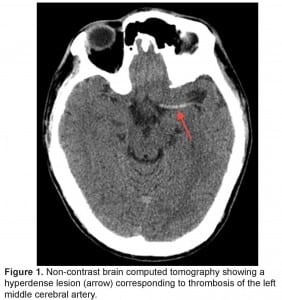
Non-contrast brain computed tomography showing a hyperdense lesion (arrow) corresponding to thrombosis of the left middle cerebral artery.
A chest, neck and brain CT angiogram was performed, which showed marked wall thickening involving the left common carotid artery with complete occlusion of the distal left common carotid artery and MCA (Figure 2). Also seen was occlusion of the right common carotid and vertebral arteries with extensive collaterals, as well as inflammatory changes surrounding the aorta and subclavian vessels. No dissection of the aorta or its branches was visualized. A brain magnetic resonance image (MRI) was performed demonstrating an evolving infarct involving the left deep basal ganglia, thalamus and cerebral cortex (Figure 3). There was restricted diffusion weighted signal of the left cerebral cortex signifying viable tissue with ongoing ischemia. As the patient had already received thrombolytic therapy and failed to improve, a neurosurgical consult was obtained and the patient was taken to the interventional suite where a thrombectomy, clot retrieval and angioplasty of the left common carotid and MCA thrombus was performed to allow for reperfusion (Figure 4).
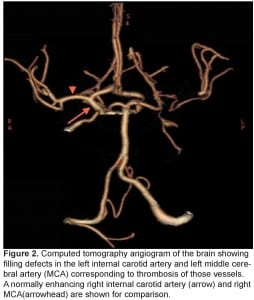
Computed tomography angiogram of the brain showing filling defects in the left internal carotid artery and left middle cerebral artery (MCA) corresponding to thrombosis of those vessels. A normally enhancing right internal carotid artery (arrow) and right MCA(arrowhead) are shown for comparison.
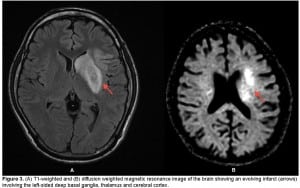
(A) T1-weighted and (B) diffusion weighted magnetic resonance image of the brain showing an evolving infarct (arrows) involving the left-sided deep basal ganglia, thalamus and cerebral cortex.
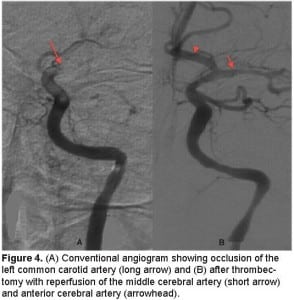
(A) Conventional angiogram showing occlusion of the left common carotid artery (long arrow) and (B) after thrombectomy with reperfusion of the middle cerebral artery (short arrow) and anterior cerebral artery (arrowhead).
Laboratory testing revealed an erythrocyte sedimentation rate (ESR) of 103 mm/hour (normal 0–15 mm/hr) and a C-reactive protein (CRP) of 51 (normal < 10). The patient’s complete blood count, chemistries, coagulation profiles, hypercoagulable studies, electrocardiogram, and chest radiograph were normal.
Given the clinical exam, laboratory results and the findings on the CT angiogram, the presumptive diagnosis of Takayasu’s arteritis was made. The patient was started on high dose steroids and methotrexate. He subsequently had normalization of his ESR and CRP and had marked clinical improvement by time of discharge.
DISCUSSION
Takayasu’s arteritis, also known as pulseless disease, is a chronic inflammatory disease of unknown etiology that affects the aorta and its main branches. It was first described by Dr. Mikito Takayasu4, a Japanese ophthalmologist, in 1905. In his report he described a 21-year-old woman with characteristic retinal arterio-venous anastomoses, syncope, and absent upper extremity pulses. Takayasu’s arteritis is most commonly seen in females between the ages of 11 and 30 and is more common in Japan, Southeast Asia, India, and Mexico. It is rare in the US with an incidence of approximately 2.6 per million per year.5
The etiology of Takayasu’s arteritis is unknown, but evidence suggests an autoimmune process, given the association with certain human leukocyte antigen (HLA) alleles and other autoimmune processes such as sarcoidosis and inflammatory bowel disease. It is also suggested that tuberculosis may have an association, given a high prevalence of active and past infection in patients with Takayasu’s arteritis.6
Takayasu’s arteritis typically presents in a pre-pulseless phase with non-specific systemic inflammatory features, such as fever, night sweats, malaise, weight loss, and arthralgias, followed by a chronic, pulseless phase with the development of vascular insufficiency and compromise. Morbidity and mortality are mainly the result of vascular occlusion and end organ damage. In the pulseless stage the typical manifestations of disease depend on the vessels involved. The most common manifestations of Takayasu’s arteritis are limb claudication and ischemia from peripheral vascular involvement, hypertension from renal artery stenosis, ophthalmologic disease as manifested by retinopathy or amaurosis fugax, aortic regurgitation resulting from dilatation of the ascending aorta, cardiac ischemia or congestive heart failure as a result of hypertensive and aortic disease, pulmonary hypertension from pulmonary arterial involvement, and lastly neurologic disease (seizures and stroke) as a result of intra and extra-cranial arterial inflammation or thrombosis. Strokes are a common complication of Takayasu’s arteritis with an estimated incidence of 10–20%.7 Stroke as the first manifestation, however, is rare and few case reports exist in the literature.
In 1990, the American College of Rheumatology proposed criteria for the diagnosis of Takayasu’s arteritis: age at disease onset ≤ 40 years, claudication of the extremities, decreased brachial artery pulse, blood pressure difference >10 mmHg in the upper extremities, bruit over subclavian arteries or aorta and arteriogram abnormalities. The presence of three out of six criteria are required for diagnosis and demonstrate a sensitivity of 90.5% and a specificity of 97.8%.8
No known serologic marker exists to diagnose or track progression of disease. ESR, the most commonly used marker to identify systemic inflammatory conditions, is not a reliable indicator and has been found to underestimate disease activity when compared to arterial histopathology or angiography.7 CT, doppler angiography and MRI/magnetic resonance angiography can aid in diagnosis and can be used to track disease progression and response to therapy.
Immediate priorities in treatment must address acute vascular compromise through either medical treatment with thrombolytics or surgical or interventional revascularization. Long term treatment is targeted at decreasing vessel inflammation and progression of vascular disease and controlling comorbid conditions. Glucocorticoids are the mainstay of anti-inflammatory treatment. Patients not responding to steroid therapy can be treated with immunomodulators, including methotrexate, cyclophosphamide and azathioprine. Despite long term treatment with corticosteroids and cytotoxic drugs, many patients will still experience progressive vascular disease. Medical management does not generally reverse pre-existing vascular stenosis or occlusion and therefore revascularization may be necessary if hemodynamically significant lesions occur.8,9 Five-year survival is estimated to be 80% and largely depends on the clinical manifestations of disease and response to medical and surgical therapy.10
Our patient had only non-specific arthralgias and headache prior to his stroke. At the time of diagnosis, he had significant disease as demonstrated by the angiographic findings on CT and angiography. He had five out of six of the criteria set forth by the American College of Rheumatology and was thus diagnosed with Takayasu’s arteritis. Treatment was started accordingly with high dose steroids and methotrexate. On discharge from the hospital, the patient had regained a significant degree of function, with 4 out of 5 motor strength on the right side and a mild residual expressive aphasia. He was discharged to an inpatient rehabilitation facility for aggressive physical, speech and occupational rehabilitation. Six months after discharge, on routine follow-up, the patient continued to have significant differential blood pressures in his upper extremities as well as bruits over his subclavian and carotid vessels. A follow-up CT angiogram of the brain and neck was performed and, despite normalization of the ESR and CRP, showed continued significant wall thickening of the subclavian and carotid arteries and aorta as well as severe re-stenosis of the left common carotid artery. The patient underwent successful stenting of the lesion. He was continued on methotrexate 25 mg weekly and prednisone 7.5 mg daily with the plan for interval CT angiograms to track the progression of vascular disease.
Footnotes
Supervising Section Editor: Rick A. McPheeters, DO
Submission history: Submitted August 18, 2011; Revision received November 9, 2011; Accepted December 2, 2011
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.12.6881
Address for Correspondence: Matthew Silver, MD, Kaiser Permanente, San Diego Medical Center, 4647 Zion Ave, San Diego, CA 92120
E-mail: matthew.a.silver@kp.org
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Neyer JR, Greenlund KJ. Prevalence of stroke—United States, 2005. MMWR. 2007 May 18;56(19):469–474. [PubMed]
2. Rosamond W, Flegal K, Friday G, et al. American Heart Association statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2007 Feb 6;115(5):e69–171. [PubMed]
3. Hart R, Miller V. Cerebral infarction in young adults: a practical approach. Stroke.1983;14:110–114. [PubMed]
4. Takayasu M. A case with peculiar changes of the retinal cerebral vessels [in Japanese]Acta Ophthal Soc Japan. 1908;12:554–555.
5. Hall S, Barr W, Lie JT, et al. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore) 1985 Mar;64(2):89–99. [PubMed]
6. Johnston SL, Lock RJ, Gompels MM. Takayasu arteritis: a review. J Clin Pathol.2002;55:481–486. [PMC free article] [PubMed]
7. Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994 Jun 1;120(11):919–29. [PubMed]
8. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990 Aug;33(8):1129–34. [PubMed]
9. Liang P, Hoffman GS. Advances in the medical and surgical treatment of Takayasu arteritis. Curr Opin Rheumatol. 2005 Jan;17(1):16–24. [PubMed]
10. Subramanyan R, Joy J, Balakrishna KJ. Natural history of aorto-arteritis.Circulation. 1989;80:429–37. [PubMed]


