| Author | Affiliation |
|---|---|
| Paul A. Willette, DO | Riverside Methodist Hospital, Mid-Ohio Emergency Services, LLC, Columbus, Ohio |
| Scott Coffield, MD | Texas A&M Health Science Center College of Medicine, Temple Clinic, Temple, Texas |
ABSTRACT
Routine urinary catheter placement may cause trauma and poses a risk of infection. Male catheterization, in particular, can be difficult, especially in patients with enlarged prostate glands or other potentially obstructive conditions in the lower urinary tract. Solutions to problematic urinary catheterization are not well known and when difficult catheterization occurs, the risk of failed catheterization and concomitant complications increase. Repeated and unsuccessful attempts at urinary catheterization induce stress and pain for the patient, injury to the urethra, potential urethral stricture requiring surgical reconstruction, and problematic subsequent catheterization. Improper insertion of catheters also can significantly increase healthcare costs due to added days of hospitalization, increased interventions, and increased complexity of follow-up evaluations. Improved techniques for catheter placement are essential for all healthcare personnel involved in the management of the patient with acute urinary retention, including attending emergency physicians who often are the first physicians to encounter such patients. Best practice methods for blind catheter placement are summarized in this review. In addition, for progressive clinical practice, an algorithm for the management of difficult urinary catheterizations that incorporates technology enabling direct visualization of the urethra during catheter insertion is presented. This algorithm will aid healthcare personnel in decision making and has the potential to improve quality of care of patients.
INTRODUCTION
Acute urinary retention (AUR) and other genitourinary conditions often lead to difficult catheterizations. Male catheterization, in particular, can be difficult, especially in patients with enlarged prostate glands or other potentially obstructive conditions in the lower urinary tract.1 Solutions to problematic urinary catheterization are not well known and when difficult catheterization occurs, the risk of failed catheterization and concomitant complications increase. Even routine urinary catheter placement may cause trauma and poses a risk of infection.1,2 Methods to reduce the incidence of infection are particularly relevant since the Centers of Medicare and Medicaid Services (CMS) under rule CMS-1533-FC no longer reimburse for catheter-associated urinary tract infections.3 The National Quality Forum, a nonprofit organization that develops national priorities and goals for performance improvement to enhance the quality of healthcare, estimated that 17% to 69% of catheter-associated urinary tract infections may be prevented with recommended infection control measures.4 Such measures could result in up to 38,000 preventable infections and 9,000 preventable deaths related to these infections per year.4 Repeated and unsuccessful attempts at blind urinary catheterization result in stress and pain for the patient, injury to the urethra, potential urethral stricture requiring surgical reconstruction, and problematic subsequent catheterization. Improper insertion of catheters also can significantly increase healthcare costs due to added days of hospitalization, increased interventions, and increased complexity of follow-up evaluations.5 Therefore, all healthcare personnel who perform urinary catheterizations should be well trained in techniques specific to managing difficult catheterizations.
Much of the work of emergency physicians involves preparing for an action-specific intervention for an illness or particular scenario. Whether airway intervention, treatment of the coagulopathic trauma patient, or managing a myocardial infarction, established protocols guide how each case should be approached. However, beyond attempting to place a Foley catheter or contacting an urologist to intervene, alternative pathways for promptly managing the complicated catheterization patient are limited. For AUR, it is not uncommon for the nursing staff to attempt placement of a urinary catheter before the emergency physician is contacted of the patient’s presence. If unsuccessful, repeated attempts with the same catheter may occur, catheterization with a catheter in a different size (typically larger) may be attempted, another catheter type may be used, or another healthcare worker may attempt the process. Such multiple attempts frequently result in injury to the urothelium, which is only 3 to 4 cell layers in thickness. In this worse-case scenario, the emergency physician is presented with a patient who has experienced multiple catheterization attempts, resulting in an iatrogenic injury. Repeated blind attempts at catheterization should be avoided to prevent escalation of a complex injury from what many consider to be a minor procedure.
Little is taught about urinary catheter placement during residency and it generally is allocated to the lowest level of training, often the medical student on the trauma service. While most emergency physicians probably never considered placement of a Foley catheter to be difficult or dangerous, what options are available when the attempt fails and the patient needs prompt relief? Another attempt may not be the best choice. Current emergency medicine teaching does not offer much guidance for managing difficult catheterizations, with urology consultation recommended when a transurethral catheter does not provide adequate bladder drainage.6 Knowledge on this topic remains sparse in both emergency medicine and nursing specialties, and recommendations seldom are supported by evidence-based research. Best practice methods for blind urinary catheter placement, based on the literature and personal experience, will be summarized in this review. In addition, for progressive clinical practice, an algorithm for the management of difficult urinary catheterizations that incorporates new technology enabling direct visualization of the urethra during catheter insertion will be presented. This algorithm will aid healthcare personnel in decision making and has the potential to improve quality of care of patients.
INITIAL CATHETERIZATION
Initial management of AUR involves prompt bladder decompression, for which there are no uniform guidelines. An initial attempt at transurethral catheterization to establish drainage is appropriate for most patients. Urethral injury, either confirmed or suspected, is an absolute contraindication to urethral catheterization.1,2 Relative contraindications include urethral stricture, recent urethral or bladder surgery, and a combative or uncooperative patient.2 Although the classic teaching triad consisting of meatal blood, distended urinary bladder with the inability to pass urine, and a high-riding prostate, raises the suspicion of urethral injury, it is infrequently reported in the medical literature and its absence should not exclude the diagnosis.7,8 A recent investigation of 46 patients by Shlamovitz and McCullough8 demonstrated that no patients with urethral or bladder injuries had a high-riding prostate, which is a clinical finding that continues to be overemphasized despite its low sensitivity for the presence of lower urinary tract injury. Retrograde urethrography is the preferred diagnostic technique to investigate injury to the urethra.7,9–11
The main types of urinary catheters used today include the Foley (self-retaining balloon), Robinson (no balloon), Coudé (curved-tip Foley with or without balloon), irrigation (3 ports), and the external Texas catheter. Size is referred to by using the French (Fr) scale (circumference in mm), in which 1 Fr equals 0.33 mm in diameter.12 An easy method of conversion between scales is to remember that each millimeter in diameter is approximately 3 Fr; therefore, an 18-Fr catheter is about 6 mm in diameter.12 Initial catheterizations most commonly are performed using a Foley catheter. The adult male urethra is typically 30 Fr and selection of a 16- or 18-Fr catheter is appropriate for most men.2,12,13 Smaller catheters (12 to 14 Fr) may be required for patients with urethral stricture, whereas patients with prostate enlargement may benefit from larger sizes (20 to 24 Fr) to avoid kinking as the catheter traverses the prostatic urethra.2 Larger catheters with irrigation capacity should be selected for patients with gross hematuria to prevent obstruction of the lumen by blood clots and subsequent urinary retention.2
Women infrequently pose a challenge for urinary catheter placement. Most issues are related to vaginal atrophy or retraction of the urethral meatus into the vagina.14 In females, shorter catheters may be used for one-time catheterizations and may prevent difficult catheterizations.12,15
Proper placement technique is critical, as failed attempts at catheterization may lead to iatrogenic injury. Forcing a catheter past the point of resistance can cause injuries ranging from a mucosal tear to more serious false passages (perforations), which are associated with infection, urethral stricture, and subsequent surgical management.1,13,15,16 In turn, urethral stricture may make future catheterizations problematic.1 The most common injury sites are the posterior and bulbous urethra.5 The most frequent injuries are false passages created by forceful catheterization, as well as mucosal and submucosal tissue tears caused by balloon inflation in an improper position in the urethra.5,14 Bleeding typically is the first sign that an injury has occurred. Besides making manual catheterization more difficult, bleeding also complicates subsequent endoscopic procedures that may be required.
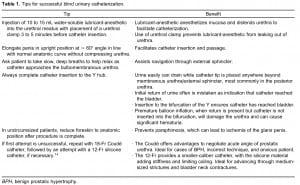
Urology consultations for catheter placement often occur when there is no organic pathology, but instead, when improper catheter placement has caused urethral injuries in patients with normal problematic anatomy.14 After analysis of data at 2 different institutions, Villanueva and Hemstreet III14 reported that 41% and 54% of patients requiring urologic consultation for difficult urinary catheterization were readily catheterized with an 18-Fr Coudé catheter. In the authors’ experience, the most common cause of difficult urinary catheterizations was an incorrect technique or benign prostatic hypertrophy (BPH) rather than urethral stricture disease.14 Before ordering a urology consultation or advancing to more complex techniques, the tips outlined in Table 1 may be beneficial in increasing the likelihood of successful passage of a urinary catheter without causing injury.1,2,5,12,14,17,18 Randomized controlled trials have established that the use of topical anesthetic gel reduces the pain associated with urethral catheterization in both men and women,19,20 although the timing of delayed versus immediate catheterization may be debatable.21 To prevent extrusion of the anesthetic gel, place a penile clamp below the glans of the penis to gently compress the pendulous urethra. Performing these steps early in the setup for catheterization allows sufficient time to provide adequate analgesia.
Suprapubic catheterization may be needed after failed attempts at transurethral catheterization or if there is pelvic or urethral trauma.11,16,22–24 It typically is indicated when there is a tight stricture in a patient who is a good candidate for urethroplasty or when a glide wire cannot be secured in the bladder owing to a completely obliterated urethra.14 Coagulopathy and active bladder cancer are contraindications.14Interventional radiologists or urologists typically perform this procedure, but it also may be performed by an emergency physician if these specialists are not available. Depending on community practice style and availability of the specialists, percutaneous suprapubic catheterization with ultrasound guidance may be easily performed at the bedside.11,14,22,25
DIFFICULT CATHETERIZATION ALGORITHM
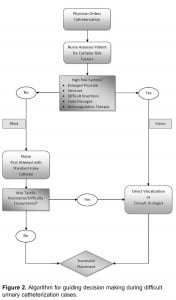
Urologic history, along with clinical observations from initial unsuccessful urethral manipulation, often provides insight into the problem that is preventing catheterization.12 For example, patients with a history of open prostatectomy may have a bladder neck contracture, whereas patients with a history of gonococcal urethritis likely will have a pendulous urethral stricture.12 A difficult catheterization scenario in the male patient is illustrated through endoscopy in Figure 1. When difficult catheterizations are encountered, the solutions provided in Table 2 may assist in obtaining successful passage without causing injury.2,5,12,14,18
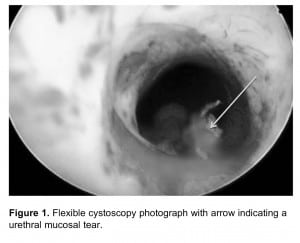
Flexible cystoscopy photograph with arrow indicating a urethral mucosal tear.
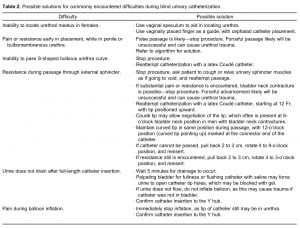
Possible solutions for commonly encountered difficulties during blind urinary catheterization.
Since ancient Greece, urinary catheters have been placed blindly.1,15 Urinary catheterization is considered an essential skill for physicians and, in the past, required surgery when blinded attempts failed. The last major advancement in urinary catheterization occurred when rubber catheters were introduced in the 18th century.15New technology now is available that enables direct visualization of the urethra while passing a catheter. One such system (DirectVision System; PercuVision, Westerville, Ohio) consists of a microendoscope that inserts into 1 lumen of a 3-way/trilumen Foley catheter. The microendoscope is connected to a camera and LED (light emitting diode), transporting light to the catheter tip and an image back to the LCD (liquid crystal display) monitor for realtime visualization of the urethra during catheter placement. Irrigation is used during catheter placement to assist in expanding the urethra, activating the lubricious coating of the catheter, and preventing debris from covering the lens at the distal tip of the catheter. A curved (Coudé) tip assists in navigating the normal S-shaped curve of the bulbous urethra. The procedure may be performed by any health professional (surgeon, physician, nurse, or allied health staff) trained to insert Foley catheters. The minimal training required to learn equipment may be incorporated into existing nursing or residency training.26
Direct visualization of the urethra enables identification of the source of resistance, obstruction, or other complication preventing blind catheter placement. Once the source of resistance is identified, a decision whether to proceed with placement under direct visualization or to stop the attempt and seek assistance may be made. In many cases, visualization may allow the source to be circumnavigated and the catheter may be successfully placed. The ultimate goal of such technology is to limit the escalation of procedures required for a successful catheterization. Although not recommended for routine catheterization, it is best used in several high-risk clinical scenarios. The algorithm depicted in Figure 2 incorporates these scenarios and may be useful in navigating difficult catheterizations.
A pilot study examined the feasibility and safety of male urinary catheterization performed by trained emergency nursing personnel using a specific visually guided device.26 Training included hands-on device practice in the hospital’s simulation center and a didactic program involving lectures on male anatomy, indications and contraindications for urinary catheter placement, and possible complications.26 Criteria for proficiency included 5 practice procedures followed by 5 successful catheterizations, as determined by one of the physician investigators.26 Among the 25 patients enrolled, there was a 100% success rate for Foley catheter placement, with minimal pain and 2 cases of gross hematuria.26 On the basis of these results, a larger prospective, randomized study comparing the visually guided device to standard male urinary catheterization is being performed.26
The National Quality Forum listed direct visualization of the urethra during insertion of catheters as one of the safe practice innovations in their safe practice guidelines for catheter-associated urinary tract infection prevention developed in 2009, recognizing that damage to the urethra can occur with blind insertion, leading to the risk of infection.4 Using direct visualization technology is in accordance with these established guidelines and benefits the patient and hospital staff. It has the potential to minimize patient pain, lower anxiety of both the patient and staff performing the catheterization, reduce iatrogenic injuries, avoid or reduce risks and costs associated with diagnostic imaging and advanced procedures, and reduce the need for urologist consultation.
Despite best efforts at problem solving, urologic consultations are necessary when the urethra cannot be entered owing to severe phimosis or meatal stenosis or if substantial resistance during catheter placement is encountered.2 If kinking occurs in the urethra and a bloody discharge is present, urethral perforation may have occurred and a urologic consultation is required. In an attempt to minimize the extent of injury to the urethra and subsequent stricture formation that may require surgical reconstruction, prompt consultation for catheter placement appears appropriate in these circumstances. Numerous advanced techniques are available to the urologist for managing difficult catheterizations.14 Blind glide wire techniques should not be performed by emergency physicians when interventional radiologists or urologists are available. Although this technique is well established in the literature, some urologists view it as controversial and consider flexible cystoscopy as the standard of care.14 In a 2011 American Urologic Association update, Villaneuva and Hemstreet III14 state that they only will perform such a technique after failure of 2 other techniques.
DISPOSITION
The stable, routine patient for whom catheterization was successful may be discharged home with urology follow-up after being fitted for a closed, leg-bag Foley system and educated about catheter management with home care. To prevent infection after catheter therapy, the integrity of the closed-catheter system should be maintained and the catheter should be removed as soon as possible. Routine prophylactic antibiotics are not necessary, as use may promote resistance and complications.2,15,27 However, 1 dose of oral antibiotic before discharge may be appropriate for certain patients,2 such as those for whom excessive manipulation of the urinary catheter occurred. The duration of catheter placement is an area of debate but typically is 1 to 7 days, depending upon patient comorbidities (ie, diabetes mellitus, ambulatory impairment, prostatic enlargement, and expectation of resolution of initial need for catheterization).24 One prospective study assessing the impact of catheter duration on voluntary voiding in men with AUR caused by BPH demonstrated that men with fluid retention volumes in excess of 1,300 mL had lower rates of failure with longer catheter duration.28 On the basis of these findings, the authors recommended longer periods of catheter placement (7 days) for this group of patients to improve the likelihood of successful voiding.28
The complicated patient with systemic illness, such as fever, hypotension, or multiple comorbid medical conditions, will require hospitalization, as will patients with complications from manipulation or decompression.16,29 An uncommon, yet frequently discussed condition, postobstructive diuresis, occurs in 0.5% to 52% of patients with chronic retention, but is not of clinical significance unless urine output of 200 mL/h occurs for more than 4 hours, which would necessitate hospitalization.16,29 Limiting the amount of urine emptying and gradual drainage are not necessary, as hematuria occurs in 2% to 16% of patients after rapid, complete bladder emptying and most likely is of little clinical significance.16,29 Hypotension occurs with bladder decompression, but again, does not seem clinically significant in otherwise healthy patients.29
Successful voiding after discharge also may be improved by pharmacologic therapy. Initiation of alpha blockers may provide sufficient decrease in smooth muscle tone at the bladder neck and in the prostate to allow successful voiding after catheter removal in men with significant prostatic enlargement, but should be used with instruction about the risk of side effects.23,24,30–35 The most common side effects are dizziness and asthenia but also can include orthostatic hypotension, headache, nasal congestion, and delayed ejaculation.23,24,30–35 Tamsulosin (0.4 mg orally daily) and alfuzosin (10 mg orally daily) are commonly used because they do not require titration dosing. Other options include phenoxybenzamine, prazosin, terazosin, and most recently, silodosin. Initiation of alpha blockers by the emergency physician is appropriate and will aid in the decision-making process of the urologist at the time of follow-up consultation regarding when to remove the urinary catheter if the patient passes a voiding trial. Patients with urinary retention after initiation of alpha blockers should undergo urologic consultation to assess therapy response and to exclude malignant disease as causative of urinary retention. Compared to placebo, long-term treatment with 5-alpha reductase inhibitors, such as dutasteride or finasteride, or a combination of finasteride and doxazosin, has been shown to produce a clinically significant reduction in total prostate volume and may prevent AUR in BPH.23,24,33,34,36–38 The use of 5-alpha reductase inhibitors combined with an alpha blocker in a single oral dose now is available. The initial use of both agents is a decision that should be made after urologic consultation when it is clear that the patient will require prostate volume reduction, justifying the addition of another agent to treat voiding dysfunction.23,34,35,37
Complicated urinary catheterization is a commonly encountered medical problem, the frequency of which is difficult to estimate.14 Patients have limited access to a clinician knowledgeable to treat this condition. Emergency physicians play a pivotal role by intervening in the acute presentation of genitourinary trauma, urinary retention, and the inability to decompress a urinary bladder. The tips and algorithm presented in this review offer guidance for improved success in blind catheter placement and also offer an advanced technique for high-risk patients by using direct visualization. Equipped with this insight, emergency medicine physicians and staff will be able to readily identify a problem with catheter placement and then have a solution available at the bedside to navigate successful catheter placement or conclude that a urologic consultation is needed. This approach offers patients better care, with less pain and complications, while minimizing hospital resources.
Footnotes
The authors thank Jodi F. Hartman, MS, for her editorial assistance in preparation of the manuscript.
Supervising Section Editor: Christopher Kang, MD
Submission history: Submitted May 27, 2011 ; Revision received August 19, 2011 ; Accepted October 31 , 2011
Full text available through open access at http://escholarship.orgfucfuciem_westjem
DOl: 10.5811fwestjem.2011.11 .6810
Address for Correspondence: Paul A. Willette, DO, Riverside Methodist Hospital, Mid-Ohio Emergency Services, LLC, 3535 Olentangy River Rd, Columbus, OH 43214
E-mail: orthopaedicresearch@yahoo.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. Dr Willette and Dr Coffield did not receive any commercial support for their work involved in preparing this manuscript. Dr Willette received honoraria from PercuVision LLC for 3 presentations involving complicated catheterizations at educational grand rounds and clinical settings. Other than the honoraria, Dr Willette has no other significant financial interests to declare. Dr Coffield does not have any significant financial interests or any declaration of conflict to report. Ms Hartman received compensation for her work in editing the manuscript.
REFERENCES
1. Hadfield-Law L. Male catheterization. Accid Emerg Nurs. 2001;9:257–263. [PubMed]
2. Thomsen TW, Setnik GS. Videos in clinical medicine: male urethral catheterization. N Engl J Med. 2006;354:e22. [PubMed]
3. Centers for Medicare and Medicaid Services Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Fed Regist.2007;72:47129–48175. [PubMed]
4. National Quality Forum Safe Practice for Better Healthcare—2009 Update: A Consensus Report. Washington DC: National Quality Forum; 2009.
5. Daneshgari F, Krugman M, Bahn A, et al. Evidence-based multidisciplinary practice: improving the safety and standards of male bladder catheterization. Medsurg Nurs.2002;11:236–246. [PubMed]
6. Policastro MA. Urinary obstruction treatment and management. WebMD LLC Web site. Available at: http://www.emedicine.medscape.com/article/778456-treatment. Accessed August 11, 2011.
7. Chapple C, Barbagli G, Jordan G, et al. Consensus statement on urethral trauma. BJU Int. 2004;93:1195–1202. [PubMed]
8. Shlamovitz GZ, McCullough L. Blind urethral catheterization in trauma patients suffering from lower urinary tract injuries. J Trauma. 2007;62:330–335. [PubMed]
9. Ingram MD, Watson SG, Skippage PL, et al. Urethral injuries after pelvic trauma: evaluation with urethrography. Radiographics. 2008;28:1631–1643. [PubMed]
10. Ramchandani P, Buckler PM. Imaging of genitourinary trauma. AJR Am J Roentgenol. 2009;192:1514–1523. [PubMed]
11. Silverman MA, Schneider RE. Urologic procedures. In: Roberts JR, Hedges JR, editors.Clinical Procedures in Emergency Medicine. 5th ed. Philadelphia, PA: Elsevier Saunders; 2010. pp. 1001–1041.
12. Carter HB, Chan DY. Basic instrumentation and cystoscopy. In: Wein AJ, Kavoussi LR, Novick AC, et al., editors. Campbell’s Urology. 9th ed. Philadelphia, PA: WB Saunders Co; 2007. pp. 161–170.
13. Gray M. Urinary retention: management in the acute care setting, part 2. Am J Nurs. 2000;100:36–44. [PubMed]
14. Villanueva C, Hemstreet GP., III Difficult catheterization: tricks of the trade. AUA Update Series. 2011;30:41–48.
15. Ramakrishnan K, Mold JW. Urinary catheters: a review. Internet J Fam Pract.2005;3:1–29.
16. Curtis LA, Dolan TS, Cespedes RD. Acute urinary retention and urinary incontinence.Emerg Med Clin North Am. 2001;19:591–619. [PubMed]
17. Newman DK. The indwelling urinary catheter: principles for best practice. J Wound Ostomy Continence Nurs. 2007;34:655–663. [PubMed]
18. Villanueva, Hemstreet GP. Difficult male urethral catheterization: a review of different approaches. Int Braz J Urol. 2008;34:401–412. [PubMed]
19. Chung C, Chu M, Paoloni R, et al. Comparison of lignocaine and water-based lubricating gels for female urethral catheterization: a randomized controlled trial. Emerg Med Australas. 2007;19:315–319. [PubMed]
20. Siderias J, Guadio F, Singer AJ. Comparison of topical anesthetics and lubricants prior to urethral catheterization in males: a randomized controlled trial. Acad Emerg Med.2004;11:703–706. [PubMed]
21. Garbutt RB, McD Taylor D, Lee V, et al. Delayed versus immediate urethral catheterization following instillation of local anaesthetic gel in men: a randomized, controlled clinical trial. Emerg Med Australas. 2008;20:328–332. [PubMed]
22. Aguilera PA, Choi T, Durham BA. Ultrasound-guided suprapubic cystostomy catheter placement in the emergency department. J Emerg Med. 2004;26:319–321. [PubMed]
23. Selius BA, Subedi R. Urinary retention in adults: diagnosis and initial management.Am Fam Physician. 2008;77:643–650. [PubMed]
24. Thomas K, Chow K, Kirby RS. Acute urinary retention: a review of the aetiology and management. Prostate Cancer Prostatic Dis. 2004;7:32–37. [PubMed]
25. Chiou RK, Morton JJ, Engelsgjerd JS, et al. Placement of large suprapubic tube using peel-away introducer. J Urol. 1995;153:1179–1181. [PubMed]
26. Willette PA, Banks KLW, Shaffer LE. Visually-guided male urinary catheterization—a feasibility study. J Emerg Nurs
27. Walker S, McGeer A, Simor AE, et al. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? CMAJ. 2000;163:273–277. [PMC free article] [PubMed]
28. Djavan B, Shahrokh S, Musbah O, et al. Does prolonged catheter drainage improve the chance of recovering voluntary voiding after acute urinary retention (AUR)? Eur Urol.1998;33(suppl 1):110.
29. Nyman MA, Schwenk NM, Silverstein MD. Management of urinary retention: rapid versus gradual decompression and risk of complications. Mayo Clin Proc. 1997;72:951–956. [PubMed]
30. Lepor H. Managing and preventing acute urinary retention. Rev Urol. 2005;7(suppl 8):S26–S33. [PMC free article] [PubMed]
31. Lucas MG, Stephenson TP, Nargund V. Tamsulosin in the management of patients in acute urinary retention from benign prostatic hyperplasia. BJU Int. 2005;95:354–357.[PubMed]
32. McNeill SA, Hargreave TB., Alfaur Study Group Alfuzosin once daily facilitates return to voiding in patients in acute urinary retention. J Urol. 2004;171(6, pt 1):2316–2320.[PubMed]
33. O’Leary MP, Roehrborn C, Andriole G, et al. Improvements in benign prostatic hyperplasia-specific quality of life with dutasteride, the novel dual 5alpha-reductase inhibitor. BJU Int. 2003;92:262–266. [PubMed]
34. Roehrborn CG. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Rev Urol.2008;10:14–25. [PMC free article] [PubMed]
35. Roehrborn CG, Schwinn DA. Alphal-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–1035.[PubMed]
36. Kaplan SA, Roehrborn CG, McConnell JD, et al. Medical Therapy of Prostatic Symptoms Research Group Long-term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in men enrolled in the MTOPS trial. J Urol. 2008;180:1030–1032.[PubMed]
37. McConnell JD, Roehrborn CG, Bautista OM, et al. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. [PubMed]
38. Roehrborn CG, Bruskewitz R, Nickel JC, et al. Proscar Long-Term Efficacy and Safety Study Group Sustained decrease in incidence of acute urinary retention and surgery with finasteride for 6 years in men with benign prostatic hyperplasia. J Urol. 2004;171:1194–1198. [PubMed]