| Author | Affiliation |
|---|---|
| Christine E. Kulstad, MD | Advocate Christ Medical Center, Department of Emergency Medicine, Oak Lawn, IL |
| Shannon C. Holt, MD | Advocate Christ Medical Center, Department of Emergency Medicine, Oak Lawn, IL |
| Aaron A. Abrahamsen, MD | Advocate Christ Medical Center, Department of Emergency Medicine, Oak Lawn, IL |
| Elise O. Lovell, MD | Advocate Christ Medical Center, Department of Emergency Medicine, Oak Lawn, IL |
ABSTRACT
Introduction:
Therapeutic hypothermia (TH) has been shown to improve survival and neurological outcome in patients resuscitated after out of hospital cardiac arrest (OHCA) from ventricular fibrillation/ventricular tachycardia (VF/VT). We evaluated the effects of using a TH protocol in a large community hospital emergency department (ED) for all patients with neurological impairment after resuscitated OHCA regardless of presenting rhythm. We hypothesized improved mortality and neurological outcomes without increased complication rates.
Methods:
Our TH protocol entails cooling to 33°C for 24 hours with an endovascular catheter. We studied patients treated with this protocol from November 2006 to November 2008. All non-pregnant, unresponsive adult patients resuscitated from any initial rhythm were included. Exclusion criteria were initial hypotension or temperature less than 30°C, trauma, primary intracranial event, and coagulopathy. Control patients treated during the 12 months before the institution of our TH protocol met the same inclusion and exclusion criteria. We recorded survival to hospital discharge, neurological status at discharge, and rates of bleeding, sepsis, pneumonia, renal failure, and dysrhythmias in the first 72 hours of treatment.
Results:
Mortality rates were 71.1% (95% CI, 56–86%) for 38 patients treated with TH and 72.3% (95% CI 59–86%) for 47 controls. In the TH group, 8% of patients (95% CI, 0–17%) had a good neurological outcome on discharge, compared to 0 (95% CI 0–8%) in the control group. In 17 patients with VF/VT treated with TH, mortality was 47% (95% CI 21–74%) and 18% (95% CI 0–38%) had good neurological outcome; in 9 control patients with VF/VT, mortality was 67% (95% CI 28–100%), and 0% (95% CI 0–30%) had good neurological outcome. The groups were well-matched with respect to sex and age. Complication rates were similar or favored the TH group.
Conclusion:
Instituting a TH protocol for OHCA patients with any presenting rhythm appears safe in a community hospital ED. A trend towards improved neurological outcome in TH patients was seen, but did not reach significance. Patients with VF appeared to derive more benefit from TH than patients with other rhythms.
INTRODUCTION
In the United States, the incidence of out-of-hospital cardiac arrest (OHCA) is increasing, with approximately 166,200 patients suffering OHCA annually.1 Survival among resuscitated patients remains low, and the majority of survivors have a poor neurological outcome. 2,3 Recently, aggressive post-resuscitation care has been recognized as an important link in the cardiac arrest chain of survival. Therapeutic hypothermia (TH) was used to treat patients resuscitated from cardiac arrest during the 1950s;4 however, complications related to the depth of cooling led to this treatment being abandoned. Positive outcomes from animal studies in the 1990s, rekindled interest in this treatment modality.5 TH is postulated to mitigate the effects of ischemia and reperfusion injury by decreasing cerebral metabolism, suppressing the release of oxygen free radicals and excitatory neurotransmitters, and by decreasing the inflammatory response. 6,7 In 2002, two randomized controlled trials evaluated the effect of mild TH on comatose patients resuscitated after ventricular fibrillation OHCA and demonstrated improvements in survival and neurological outcome. 8, 9 Currently, both the International Liaison Committee on Resuscitation (ILCOR) and the American Heart Association (AHA) recommend the use of TH in the treatment of persistently comatose patients resuscitated after ventricular fibrillation OHCA.10, 11
A number of studies published since 2002 support the use of TH after cardiac arrest, but only one was conducted solely at a community hospital.12–18 Also, few data have been published on patients treated with TH after presenting with rhythms other than non-perfusing ventricular fibrillation or ventricular tachycardia (VF/VT); consequently, firm conclusions cannot be drawn about the benefits of TH in these other populations.9, 19–22
In November 2006, we began to treat comatose patients resuscitated from OHCA with TH, regardless of their presenting rhythm. We hypothesized that establishing this protocol in our community hospital emergency department (ED) would decrease mortality and improve neurological outcome in these patients without increasing complication rates. The primary aim of our study was to assess the impact of our TH protocol on in-hospital mortality by comparing the mortality rates of treated patients with those of control patients from the preceding 12 months. Secondarily, we evaluated the neurological status upon hospital discharge and complication rates of both groups.
METHODS
Study Design
We conducted a retrospective, observational study of the mortality of patients resuscitated from OHCA in our ED from November 2006 through November 2008. The control group consisted of patients treated in our ED during the preceding 12 months, prior to the implementation of our TH protocol. This study was approved by the hospital’s Institutional Review Board, with a waiver of informed consent.
Study Setting and Population
This study was conducted at a large tertiary care suburban community hospital with over 85,000 ED visits annually and nearly 700 inpatient beds.
Study Protocol
For our TH protocol we use an endovascular cooling catheter (ICY Catheter, IC-3893, Alsius, Irvine CA) with the goal to cool the patient to a target temperature of 33 °C within four hours. The Alsius ICY catheter is only intended for placement in the femoral vein. No patients were given cold intravenous saline. Our protocol advises using ice packs if the target temperature is not reached in four hours, but their use was not routinely noted in the medical record. Temperatures are monitored with a rectal or esophageal temperature probe. Patients are cooled for 24 hours from the onset of cooling, and then actively re-warmed at a rate of 0.5 °C/hour to a goal temperature of 36.5 °C using the endovascular catheter. Shivering is prevented with sedative medications, such as propofol, lorazepam and fentanyl; paralytics are added only if sedatives are ineffective. Vital signs are measured every 30 minutes until the goal temperature is reached and then every two hours. At the onset of cooling and at eight and 16 hours, blood tests and a 12-lead ECG is performed. The blood tests include a complete blood count, metabolic panels, coagulation studies, cardiac enzymes and arterial blood gas. Additionally, two sets of blood cultures are obtained at eight hours. Other testing and treatments are at the discretion of the treating physicians. Patients treated with TH are eligible for percutaneous coronary intervention (PCI) and anti-coagulation. Decisions regarding withdrawal of care are made by the primary physician or intensivist, usually in consult with a neurologist, but do not follow a standardized protocol. Study investigators are not involved in end-of-life decisions.
Patients were eligible for treatment with TH if they were 18 years of age or older and remained unresponsive to verbal stimuli following return of spontaneous circulation (ROSC) after cardiac arrest. Patients with any initial rhythm and with witnessed or unwitnessed arrest were eligible. Exclusion criteria were pregnancy, a systolic blood pressure less than 90mmHg despite the use of vasopressors, traumatic injuries, an initial temperature less than 30°C, a primary intracranial event determined by physician judgment, and known pre-existing coagulopathy. Eligible patients were treated with TH at the discretion of the treating physician.
We identified patients primarily by searching records from our ED automated medication and supply management machine for documentation that an endovascular cooling catheter had been dispensed. To ensure that we located all eligible patients, we also searched the diagnosis field of our ED’s electronic medical record (EMR) using the key words: arrest, vfib, vtach, fibrillation, ventricular, asystole, and PEA (pulseless electrical activity).
We identified patients in our control group by searching our EMR diagnosis field for the same keywords. The charts extracted were reviewed to determine if the patients treated before the institution of our TH protocol met the same inclusion and exclusion criteria as our study group and survived to hospital admission.
Measurements
We created a standardized abstraction form for data collection prior to the start of the study. The data collected included patient demographics, hospital length of stay (LOS), survival to hospital discharge, initial recorded arrest rhythm, and neurological status at discharge using the Glasgow-Pittsburgh Cerebral Performance Category (CPC). We determined the CPC by chart review. Abstractors were not blinded to the patient’s treatment group if it was specified in the inpatient chart. We also recorded complications during the first 72 hours, using pre-determined definitions. These included the rate of significant bleeding (requiring transfusion, or surgical or gastroenterological consultation), sepsis (meeting systemic inflammatory response syndrome criteria plus documented infection or positive culture), pneumonia (infiltrate on chest radiograph or clinical diagnosis recorded on chart), renal failure (new use of dialysis or continuous renal replacement therapy), and arrhythmias (requiring medical or electrical therapy) in the first 72 hours.
Chart abstractors met at the start of the study to define methods and were unaware of patient outcomes when abstracting data from ED records.
Our primary outcome was in-hospital mortality. Our secondary outcomes were neurological status on hospital discharge and complication rates.
Data Analysis
For the study group, our analysis of outcomes includes all patients for whom we initiated our TH protocol, regardless of whether the target temperature was reached, cooling was halted, or the patient died before hospital admission. We did not include patients who had ROSC during the study period but did not have TH initiated, even if they met inclusion and exclusion criteria. Demographic and clinical characteristics are described by means with 95% confidence intervals (CI) for normally distributed data and by medians with interquartile range (IQR) for non-normal data. We compared the mortality of each of the two groups with 95% CI, and compared the unadjusted mortality between the two cohorts with the χ2 test. We considered values of p < 0.05 to be statistically significant. Analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL).
RESULTS
Seventy-two patients with ROSC survived to hospital admission during our study period. Of these, 34 were eligible for TH but were not cooled for the following reasons: catheter could not be placed (n=7), co-existing infection (n=4), poor baseline health (n=4), deemed too unstable (n=3), do-not-resuscitate status (n=2), cooling unit in use for another patient (n=1), various (n=6) or unrecorded reasons (n=6). Reasons for catheter placement failure included the large size of the catheter, thrombus in the vein, contractures, skin breakdown and body habitus. Of the six patients with various reasons recorded, two had respiratory arrest followed by cardiac arrest, which likely was interpreted as a contraindication for TH; one had pulmonary embolism; one had suspected aortic dissection; one was transported for emergent PCI before the TH catheter was placed; and one patient was not treated due to “unknown baseline mental status.”
The remaining 38 patients were treated with our TH protocol. We were unable to determine time-to-target temperature in 13 patients (34.2%), either because the patient’s temperature was not recorded after the cooling catheter was placed or because the time of initiation of TH was not clearly documented. In the remaining 25 patients the median time to reach the target temperature was 240 minutes (IQR 115 min to 405 min).
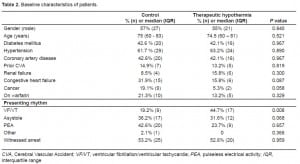
For patients treated with TH, the mortality rate was 71.1% (95% CI 56% to 86%) with an odds ratio of 0.94 (95% CI 0.36 to 2.42). Eight percent (95% CI 0% to 17%) had a good neurological outcome (defined as a Glasgow-Pittsburgh CPC score of 1 or 2) on hospital discharge. The initial documented rhythms in this group were VF/VT in 44.7% (n=17), PEA in 31.6% (n=12), and asystole in 23.7% (n=9). The arrest was witnessed in 20 patients (52.6%). The median age was 74.5 (IQR 60 – 81) years; the median hospital LOS was 4.5 (IQR 2 – 11.5) days; and 55% were male (Tables 1 and and 2). Five patients (13.2%) did not complete the protocol.
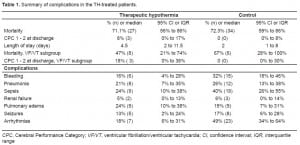
Complications in the TH-treated patients were as follows: bleeding in six [16% (95% CI 4% to 28%)]; pneumonia in eight [21% (95% CI 7% to 35%)]; sepsis in nine [24% (95% CI 10% to 38%)]; renal failure in two [5% (95% CI 0% to 13%)];arrhythmias in seven [18% (95% CI 6% to 31%)]; and seizures in five [13% (95% CI 2% to 24%)] (Table 1).
Our control group included 47 patients, with a mortality rate of 72.3% (95% CI 59% to 86%). None (95% CI 0% to 8%) had a good neurological outcome on hospital discharge. The presenting rhythms of the control group were VF/VT in 19.2% (n=9), PEA in 36.2% (n=17), asystole in 42.6% (n=20), and 2.1% documented as slow wide complex (n=1). Cardiac arrest was witnessed in 25 (53.2%) patients. The median age was 75 (IQR 60 to 83) years, the median hospital LOS was two (IQR 1 to 8) days, and 57% were male (Tables 1 and and 2).
Complications in the control group were as follows: bleeding in 15 [32% (95% CI 18% to 46%)]; pneumonia in 12 [26% (95% CI 13% to 38%)]; sepsis in 19 [40% (95% CI 26% to 55%)]; renal failure in three [6% (95% CI 0% to 14%)]; arrhythmias in 23 [49% (95% CI 34% to 64%)]; and seizures in eight [17% (95% CI 6% to 28%)] (Table 1).
Of the 17 patients treated with TH whose initial documented rhythm was VF/VT, mortality was 47% (95% CI 21% to 74%). We observed good neurological outcome in 18% of these patients (95% CI 0% to 38%). Of the nine in the control group with an initial documented rhythm of VF/VT, mortality was 67% (95% CI 28% to 100%). Good neurological outcome was seen in none of these patients (95% CI 0% to 30%) (Table 1).
The mortality rate for all 72 patients who had ROSC and survived to hospital admission during the study period was 70.8% (95% CI 59% to 80%). The 34 patients who met the inclusion and exclusion criteria for TH but were not treated had a mortality rate of 70.6% (95% CI 17% to 46%). Four of these 34 patients who were eligible but not treated with TH had an initial documented rhythm of VF/VT.
DISCUSSION
The mortality rate of patients treated with our TH protocol was not significantly different from that of our control patients; however, we did find a non-statistically significant trend towards improved neurological outcomes in the TH group. Complication rates were also not significantly different between the two groups, although there was a trend towards more bleeding, sepsis and arrhythmias in the control group. In patients with an initial rhythm of VF/VT, there was a trend towards improved mortality and neurological outcomes in the group treated with TH.
The number of published studies supporting the use of TH in the setting of resuscitated cardiac arrest continues to grow. 12–15, 16, 17 A recent review of studies of patients treated with TH after ROSC from any presenting rhythm concluded that its use improved survival and favorable neurological outcome with an odds ratio of 2.5 for both measures; of note, only one of the included studies was performed at a community hospital.23 In other studies including all rhythms, much of the survival and neurological outcome benefit was limited to patients presenting with VF/VT cardiac arrest. 16,17, 24 However, a recent study by Nielsen et al. reported a more dramatic effect of TH in non- VF/VT rhythms. Twenty-one percent of patients with an initial rhythm of asystole and 22% with PEA were discharged with good neurological outcome.25
Our overall survival rates and numbers of patients discharged with favourable outcomes were low compared to the landmark trials by Bernard and the HACA group.8, 9 Unlike those trials, we included patients with any presenting rhythm as well as patients with unwitnessed cardiac arrest. In addition, the median age of our patients was 10–15 years greater. 8, 9 All of these factors would be expected to lower survival rates.
Some decrease in the rate of favourable outcomes is not uncommon when a therapy is initially studied in a community hospital setting. 24 The one other published study performed at a community hospital demonstrated a lower mortality rate (61%) and higher rate of discharge with good neurological outcome (33%).18 Their study population was younger (mean age of 62 compared to a median age of 75); they did not specify presenting rhythms and used a different neurological outcome scale. These factors limit the ability to compare the outcomes between studies.
Although our study did not find a statistically significant benefit from TH, our sample size was small, increasing the likelihood of a type 2 error. The percentage of patients presenting with VF/VT was low in our study compared to others that included all rhythms, which may have depressed the expected treatment benefit.16, 17, 24 In patients with VF/VT, where the evidence of benefit from TH is stronger, our point estimates show an improvement in the incidence of good neurological outcome from 0 to 18%. If this effect was borne out with a larger sample size, the use of a TH protocol would be justified. The published evidence of benefit for rhythms other than VF/VT is not as robust, and none was found in our study.
ILCOR and the AHA recommend the use of TH in unresponsive patients resuscitated after ventricular fibrillation OHCA. It is estimated that an additional 2,298 patients per year in the United States would have a good neurological outcome if TH was fully implemented in comatose survivors of OHCA.26 Despite this recommendation, TH continues to be underused. In a 2005 survey of emergency physicians, cardiologists and critical care specialists involved in post-resuscitation care, 74% of United States respondents had never used TH.27 Commonly cited reasons for not using TH included “not enough data”, “not part of Advanced Cardiac Life Support Guidelines”, “have not considered cooling therapy”, and “too technically difficult to use.”27 In developing our TH protocol, we encountered these same barriers to implementation despite significant educational efforts for our physicians and nurses. In the present study, 14 of 34 potentially eligible patients were not treated with TH for reasons that were not clearly documented, and seven of the 34 were not cooled because placement of the endovascular catheter was unsuccessful.
LIMITATIONS
There are a number of limitations to our study, including its being limited to a single institution with a small sample size. We chose to evaluate in-hospital mortality and neurological outcome at the time of hospital discharge rather than longer-term survival and disability. We used the Glasgow-Pittsburgh CPC as our neurological outcome measure. The CPC has been criticized for being a relatively gross assessment tool;28 however, it still is a standard outcome measure used in resuscitation research. Our protocol and study design designated the use of an endovascular catheter for cooling, thus limiting our ability to generalize our results to institutions using alternative cooling techniques. The 2005 AHA guidelines for emergency cardiovascular care were published before the period of our historical controls, and no other significant changes in ED resuscitation were implemented during our study. However, the medical and cardiac intensive care units (ICUs) at our hospital became closed units in June 2008, which may have impacted the care of TH patients in the ICU as compared with historical controls. Our hospital does not have a standardized protocol for comprehensive post-resuscitation care, although some protocols, including emergent PCI and maintenance of euglycemia, do exist.
Although our control and study groups did not differ statistically with regard to baseline characteristics, the control group did contain more patients with congestive heart failure and cancer. In addition, there were statistically fewer control patients who presented with VF/VT as opposed to other rhythms in comparison to the study population. Both factors likely favor the group treated with TH.
CONCLUSION
Although we demonstrated a trend towards improved neurologic outcomes in patients treated with TH as compared with historical controls, we found no overall difference in mortality. In patients with an initial rhythm of VF/VT, those treated with TH showed a trend towards improved mortality and neurologic outcomes. Our TH protocol appears safe, as we found no significant difference in complication rates between patients treated with TH and historical controls. Large collaborative descriptive studies of TH are now needed especially involving non-university institutions and patients with presenting rhythms other than VF/VT.
Footnotes
Supervising Section Editor: David E. Slattery, MD
Submission history: Submitted September 4, 2009; Revision Received February 7, 2010; Accepted March 1, 2010
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Christine E. Kulstad, MD, Department of Emergency Medicine, Advocate Christ Medical Center, 4440 W. 95th St. Oak Lawn, IL 60453
Email ckulstad@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. American Heart Association: Heart Disease and Stroke Statistics. Dallas, TX: 2008.
2. Madl C, Holzer M. Brain function after resuscitation from cardiac arrest. Curr Opin Crit Care. 2004 Jun;10:213–7. [PubMed]
3. Herlitz J, Bahr J, Fischer M, et al. Resuscitation in Europe: a tale of five European regions.Resuscit. 1999 Jul;41:121–31.
4. Benson DW, Williams GR, Jr, Spencer FC, et al. The use of hypothermia after cardiac arrest. Anesth and analg. 1959 Nov–Dec;38:423–8.
5. Safar P. Resuscitation from clinical death: pathophysiologic limits and therapeutic potentials. Crit Care Med. 1988 Oct;16:923–41. [PubMed]
6. Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscit. 2006 Sep;70:369–80.
7. Varon J, Acosta P. Therapeutic hypothermia: past, present, and future. Chest. 2008 May;133:1267–74. [PubMed]
8. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002 Feb 21;346:557–63. [PubMed]
9. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002 Feb 21;346:549–56. [PubMed]
10. Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003 Jul 8;108:18–121.
11. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circul. 2005 Dec 13;112:IV1–203.
12. Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscit. 2007 Apr;73:29–39.
13. Holzer M, Bernard SA, Hachimi-Idrissi S, et al. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005 Feb;33:414–8. [PubMed]
14. Busch M, Soreide E, Lossius HM, et al. Rapid implementation of therapeutic hypothermia in comatose out-of-hospital cardiac arrest survivors. Acta anaesthesiologica Scandinavica. 2006 Nov;50:1277–83. [PubMed]
15. Belliard G, Catez E, Charron C, et al. Efficacy of therapeutic hypothermia after out-of-hospital cardiac arrest due to ventricular fibrillation. Resuscit. 2007 Nov;75:252–9.
16. Oddo M, Schaller MD, Feihl F, et al. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006 Jul;34:1865–73. [PubMed]
17. Bro-Jeppesen J, Kjaergaard J, Horsted TI, et al. The impact of therapeutic hypothermia on neurological function and quality of life after cardiac arrest. Resuscit. 2009 Feb;80:171–6.
18. Scott BD, Hogue T, Fixley MS, et al. Induced hypothermia following out-of-hospital cardiac arrest; initial experience in a community hospital. Clin Card. 2006 Dec;29:525–9.
19. Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med.2007 Apr;35:1041–7. [PubMed]
20. Oddo M, Ribordy V, Feihl F, et al. Early predictors of outcome in comatose survivors of ventricular fibrillation and non-ventricular fibrillation cardiac arrest treated with hypothermia: a prospective study. Crit Care Med. 2008 Aug;36:2296–301. [PubMed]
21. Hachimi-Idrissi S, Corne L, Ebinger G, et al. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscit. 2001 Dec;51:275–81.
22. Kim F, Olsufka M, Longstreth WT, Jr, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circul. 2007 Jun 19;115:3064–70.
23. Sagalyn E, Band RA, Gaieski DF, et al. Therapeutic hypothermia after cardiac arrest in clinical practice: review and compilation of recent experiences. Crit Care Med. 2009 Jul;37:S223–6.[PubMed]
24. Hay AW, Swann DG, Bell K, et al. Therapeutic hypothermia in comatose patients after out-of-hospital cardiac arrest. Anaesthesia. 2008 Jan;63:15–19. [PubMed]
25. Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta anaesthesiologica Scandinavica. 2009 Aug;53:926–34. [PubMed]
26. Majersik JJ, Silbergleit R, Meurer WJ, et al. Public health impact of full implementation of therapeutic hypothermia after cardiac arrest. Resuscit. 2008 May;77:189–94.
27. Merchant RM, Soar J, Skrifvars MB, et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006 Jul;34:1935–40. [PubMed]
28. Raina KD, Callaway C, Rittenberger JC, et al. Neurological and functional status following cardiac arrest: method and tool utility. Resuscit. 2008 Nov;79:249–56.


