| Author | Affiliation |
|---|---|
| Matías José Fosco, MD | Zubizarreta Hospital, Department of Emergency Medicine, Buenos Aires, Argentina |
| Victoria Ceretti, MD | Zubizarreta Hospital, Department of Emergency Medicine, Buenos Aires, Argentina |
| Daniel Agranatti, MD | Zubizarreta Hospital, Department of Emergency Medicine, Buenos Aires, Argentina |
ABSTRACT
Introduction:
High levels of inflammatory biochemical markers are associated with an increased risk among patients with acute coronary syndrome (ACS). The objective of the current study was to evaluate the prognostic significance of the systemic inflammatory response syndrome (SIRS) among ACS patients with no clinical or radiological evidence of congestive heart failure (CHF).
Methods:
Consecutive patients with ACS and no clinical or radiological evidence of CHF in the emergency department (ED) were included in the study. The endpoint was hospital mortality. Categorical variables were compared by calculating proportions with 95% confidence intervals (CIs) and by using the Fisher Exact test. Continuous variables were compared by using the Wilcoxon Rank Sum test. The association of the variables with hospital mortality was assessed by using the logistic regression analysis.
Results:
The study included 196 patients (60 years; female 32.6 %). Six patients (3.1 %) died in hospital and 22 patients (11.2 %) had SIRS on admission to the ED. The following variables were predictors of hospital mortality: age with an odds ratio (OR) of 1.1 (95% CI, 1–1.2) for each one additional year (p <0.01), systolic arterial pressure with an OR 0.9 (95% CI, 0.9–1), diastolic arterial pressure with an OR 0.9 (95% CI, 0.8–1) for each one additional mmHg (p < 0.01), respiratory rate with an OR 1.5 (95% CI, 1.2–1.9) for each one additional breath per minute (p < 0.01), and SIRS with an OR 9 (95% CI, 1.7–47.8) (p 0.02). Because of the small number of events, it was not possible to assess the independence of these risk factors.
Conclusion:
SIRS was a marker of increased risk of hospital mortality among patients with ACS and no clinical or radiological evidence of CHF.
INTRODUCTION
There is increasing evidence supporting the pathogenic role of inflammation in acute coronary syndrome (ACS).1–4 The local inflammatory process at the coronary artery plaque may cause the release of cytokines and other inflammatory acute-phase reactants into the circulation.5 Indeed, some evidence suggests that an independent systemic inflammatory process, apart from the local one, may also be involved in the pathogenesis of ACS.6 Clinical manifestation of systemic inflammation is known as systemic inflammatory response syndrome (SIRS), which may be seen in infections and a variety of other conditions.7,8 The diagnosis of SIRS is based on heart rate, respiratory rate, body temperature, and leukocyte count.7
Effective triaging of ACS patients is one of the main subjects of investigation in emergency medicine. One investigation line focuses on the subjacent inflammatory process as a prognostic factor. It has been demonstrated that high plasma levels of inflammatory biochemical markers are associated with an increased risk of major cardiac events in ACS patients.5, 9–11 However, while these biochemical markers are not routinely available in the emergency department (ED), SIRS may be easily assessed in almost every ACS patient. We hypothesized that SIRS could be a prognostic marker among ACS patients. Since tachycardia and tachypnea, two of the diagnostic criteria of SIRS, are strongly associated with congestive heart failure (CHF), 12 we excluded ACS patients with clinical or radiological evidence of CHF.
The objective of the current study was to evaluate SIRS in the ED as a predictor of hospital mortality among ACS patients with no clinical or radiological signs of CHF.
METHODS
Study design
This prospective cohort study included ACS patients consecutively admitted to the ED between February 2003 and January 2004. The study was approved by the local Institutional Research Board. The outcome was hospital mortality.
Study setting and population
The study was conducted in an urban teaching hospital with 13 ED beds. The ED sees more than 86,000 patients per year. Consecutive patients aged more than 21 years old with confirmed diagnosis of ACS were enrolled in the study. All patients provided an informed consent. Patients with clinical or radiological signs of CHF were excluded from the study.
Study protocol
Medical history, physical exam, a 12-lead electrocardiogram, leukocyte count in peripheral blood and a chest radiograph were performed in every patient. The electrocardiogram was repeated in case of recurrent symptoms. Leukocytes were counted by using an automated cell counter as per standard laboratory techniques. Each patient had two or more determinations of plasma cardiac troponin I, one of them performed at least 12 hours after the onset of the symptoms. Cardiac troponin I concentrations were measured by chemiluminescence assay, using an ACS: 180 automated analyzer (Bayer Diagnostics™) with a detection limit of 0.1 ng/ml and a cut-off value for myocardial necrosis of 0.5 ng/ml. Other diagnostic procedure and therapeutic strategies were decided by the medical team in charge of the patient. Data collection forms included medical history, clinical examination on admission to the ED and complementary tests (laboratory assays, stress test, myocardial perfusion test or coronary angiography) performed during hospitalization.
SIRS was defined by the presence of at least two of the following criteria: 1) heart rate >90 beats/minute, 2) respiratory rate >20 breaths/minute, 3) body temperature >38°C or <36°C, and 4) leukocyte blood count >12 x 103/mm3 or < 4 x 103/mm3.
The final diagnosis of acute myocardial infarction (AMI), unstable angina (UA), and CHF were independently assigned by two cardiologists based on the following definitions.
A final diagnosis of AMI was confirmed in the presence of two or more measurements of plasma cardiac troponin I above the cut-off value for myocardial necrosis (>0.5 ng/ml).
A final diagnosis of UA was made in the presence of at least one of the following criteria: 1) two or more determinations of plasma cardiac troponin I within the range of myocardial injury (0.1 – 0.5 ng/ml), 2) ischemic abnormalities in at least two contiguous leads on the initial electrocardiogram (transient ST-segment depression ≥0.5 mm, transient ST-segment elevation ≥1 mm, or T-wave inversion ≥2 mm), or 3) any evidence of severe coronary artery disease on complementary studies performed during hospitalization (a positive exercise stress test or cardiac perfusion test, or a coronary angiography demonstrating any severe stenosis in a major branch).
The diagnosis of CHF was based on physical exam reports (jugular venous distension, third sound, or pulmonary rales) and initial chest radiography (pulmonary edema). Echocardiography was not available in the ED.
In case of disagreement, a third cardiologist determined the final diagnosis. Inter-rater agreement was not evaluated.
Data analysis
On the basis of a previous report,13 120 patients were required to detect a 2.9% (95% confidence interval [CI], 0–5.9%) of hospital mortality rate among ACS patients with no CHF on admission to the hospital.
Categorical variables were reported by using proportions and continuous variables by using medians and interquartile range (IR). Categorical variables were compared by calculating proportions with 95% CIs and by using the Fisher Exact test. Continuous variables were compared by using the Wilcoxon Rank Sum test. Abnormal values for body temperature and leukocyte count draw a U-shaped curve; therefore they were analyzed as dichotomized variables (“normal”/”abnormal”). The logistic regression analysis was used to determine how factors predicted hospital mortality. The odds ratios (ORs) for in-hospital mortality and the 95% CIs were derived by using the asymptotic standard error of the estimate. Software package Excel™ version 2000 (Microsoft™ Corporation, 1999) was used for data base management and Statistix™ version 7.0 (Analytical Software™) was used for all calculations.
RESULTS
Description of the population
During the study period 255 ACS patients were evaluated in the ED. 59 (23.1%) patients had clinical or radiological signs of CHF and were excluded. The study population comprised of 196 patients (76.9%). Population characteristics are shown in Table 1. The final diagnosis was AMI in 73 patients (37.2%) and UA in 123 patients (62.8%).
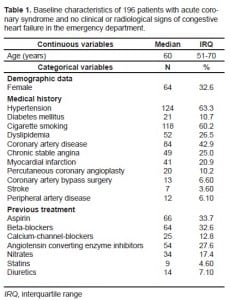
Main results
Six patients (3.1%) died in hospital. The comparison between survivors and non-survivors is shown in Table 2. The variables age, systolic and diastolic blood pressure, ST-segment elevation, and SIRS demonstrated a statistically significant difference between survivors and non-survivors. Because of the small number of events, it was not possible to assess the independence of these risk factors.14
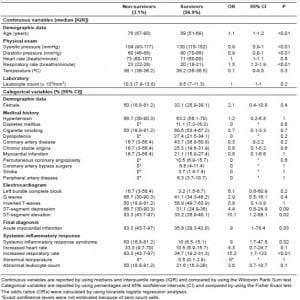
22 patients (11.2%) had SIRS. The mortality rate was 13.6% (95% CI, 0–28) among patients with SIRS and 1.7% (95% CI, 0–3.7) among patients without SIRS [risk ratio (RR) 7.9, 95% CI, 1.7–36.8] (p < 0.01). The AMI rate was not statistically different between both groups of patients: 40.9% (95% CI, 20.3–61.3) among patients with SIRS and 36.8% (95% CI, 29.6–43.9) among patients without SIRS (RR 1.1, 95% CI, 0.6–1.9) (p 0.7).
DISCUSSION
In the current study, ACS patients with SIRS on admission to the ED were at an increased risk of hospital mortality, compared with ACS patients without SIRS. SIRS was a predictor of mortality along with traditional risk factors, such as age, blood pressure, or ST-segment elevation.
Inflammation plays a central role in the development of atherosclerosis and in the process of plaque rupture in ACS.15 Acute phase reactants of inflammation may increase in plasma, which has been shown to provide prognostic information among ACS patients.16
SIRS may be caused by the activation of the immune system8 in patients with an infectious disease;17–19 however, SIRS can develop in other non-infectious conditions, including traumatic injuries,20,21 critical surgeries,22,23 burns,20 or pancreatitis.24 To our knowledge, the current study is the first to evaluate the prevalence and prognostic significance of SIRS among ACS patients. In a previous study, Rangel-Frausto et al25 showed that the prevalence of SIRS in a general ED was 25 to 64%. In our study, only 11.2% of patients had SIRS. However, prevalence of SIRS in our study might have been higher if patients with CHF had not been excluded because tachycardia and tachypnea are common clinical manifestations in this condition.12 Rangel-Frausto el al 25 reported that 32 to 64% of patients with SIRS developed sepsis during hospitalization. Patients in our study did not develop any infectious disease within 72 hours after admission to the hospital; therefore, infection did not appear to have been involved in the pathogenesis of SIRS among our patients. ACS patients with SIRS were at an increased risk for hospital mortality. This finding is consistent with the findings of previous studies, which showed that SIRS was a marker of increased risk of death among patients with intestinal bleeding,18 critical surgeries23 and acute pancreatitis.24
Two of the components of SIRS, tachycardia26–30 and high leukocyte count,31–33 are well-known markers of risk in ACS patients.
In summary, SIRS may contribute to stratify the risk of ACS patients with no clinical or radiological signs of CHF in the ED.
LIMITATIONS
This study has important limitations. First, the standard definition of SIRS is strict and excludes patients with mild distortion of the inflammatory parameters.34 Moreover, the biochemical markers of inflammation other than leukocytes, such as C-reactive protein, fibrinogen, interleukin-6, tumor necrosis factor α may be more accurate for establishing an inflammatory state than the measurement of non-specific clinical parameters.8 However, the objective of this study was to evaluate the prognostic significance of classical SIRS, which may be easily determined in the ED setting.
Second, it could be hypothesized that tachypnea and tachycardia had been subtle signs of CHF, 12,35 a marker of increased risk among ACS patients.13, 36–39 We could not strictly evaluate this hypothesis because other complementary studies, such as echocardiography or plasma B-type natriuretic factor,39 were not available in the ED.
Third, the increase in heart or respiratory rate may have been associated with fear or anxiety, 40which are frequently triggered by pain.41 Consequently, it is possible to speculate that persistent chest pain, an ACS risk marker, 42 rather than inflammation was the cause of tachycardia and tachypnea in our study. However, in a clinical setting, Marco et al43 did not identify any significant association between pain score and vital signs among more than 1000 ED patients, including 80 with AMI.
Fourth, the frequency of abnormal body temperature was low in our study (only one patient), despite the fact that fever has been described as a common finding after an AMI.44 Previous studies45–48 reported serial body temperature determinations among AMI patients, while our study reported only the first determination in the ED among patients across all ACS subsets. Gabriel et al49 attributed the fever to a systemic inflammatory response, a conclusion supported by the concomitant rise of acute phase reactants.
Finally, the independence of the risk factors could not be assessed because the number of events was small.14
CONCLUSION
The presence of SIRS on the admission to the ED was a marker of increased risk of hospital mortality among ACS patients with no clinical or radiological evidence of CHF.
Footnotes
Supervising Section Editor: Matthew Strehlow, MD
Submission history: Submitted March 24, 2009; Revision Received November 17, 2009; Accepted March 20, 2010
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Matías José Fosco, MD, Department of Emergency Medicine, Zubizarreta Hospital, Nueva York 3952, (1419) Buenos Aires, Argentina
Email: mjfosco@ffavaloro.org
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Van der Wal AC, Becker AE, van der Loos CS, et al. Site of initial rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant 315 plaque morphology. Circulation. 1994;89:36–44. [PubMed]
2. Moreno PR, Falk E, Palacios IF, et al. Macrophage infiltration in acute coronary syndromes: implications for plaque rupture. Circulation. 1994;90:775–8. [PubMed]
3. Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1084–8. [PubMed]
4. Mach F, Schönbeck U, Bonnefoy JY, et al. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–9. [PubMed]
5. Liuzzo G, Biasucci LM, Rebuzzi AG, et al. Plasma protein acute-phase response in unstable angina is not induced by ischemic injury. Circulation. 1996;94:2373–80. [PubMed]
6. Takeshita S, Isshiki T, Ochiai M, et al. Systemic inflammatory responses in acute coronary syndrome: increased activity observed in polymorphonuclear leukocytes but not T lymphocytes.Atherosclerosis. 1997;135:187–332. [PubMed]
7. Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee: American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed]
8. Levy MM, Fink MP, Marshall JC, et al. For the International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med.2003;31:1250–6. [PubMed]
9. Liuzzo G, Biassucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–24. [PubMed]
10. Biassucci LM, Vitelli A, Liuzzo G, et al. Elevated levels of interleukin-6 in unstable angina.Circulation. 1996;94:874–7. [PubMed]
11. Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. N Engl J Med. 2000;343:1139–47.[PubMed]
12. Nieminen MS, Brutsaert D, Dickstein K, et al. on behalf of EuroHeart Survey Investigators EuroHeart failure Survey II (EHFS): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36. [PubMed]
13. Steg PG, Dabbous OH, Feldman LJ, et al. for the Global Registry of Acute Coronary Events (GRACE) Investigators Determinants and prognostic impact of heart failure complicating acute coronary syndromes. Observations from the Global Registry of Acute Coronary Events (GRACE)Circulation. 2004;109:494–9. [PubMed]
14. Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. [PubMed]
15. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med.2005;352:1685–95. [PubMed]
16. Newby LK. Markers of cardiac ischemia, injury, and inflammation. Prog in Cardiovasc Dis.2004;46:404–16.
17. Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units; French ICU Group for Severe Sepsis. JAMA. 1995;274:968–74. [PubMed]
18. Affesa B. Systemic inflammatory response in patients hospitalized for gastrointestinal bleeding.Crit Care Med. 1999;27:554–7. [PubMed]
19. Afessa B, Green B, Delke I, et al. Systemic inflammatory response syndrome, organ failure, and outcome in critically ill obstetric patients treated in an ICU. Chest. 2001;120:1271–7. [PubMed]
20. Kowal-Vern A, Sharp-Pucci MM, Wallenga JM, et al. Trauma and thermal injury: comparison of hemostatic and cytokine changes in the acute phase of injury. J Trauma. 1998;44:325–9. [PubMed]
21. Harwood PJ, Gainnoudis PV, Van Griensen, et al. Alterations in the systemic inflammatory response after early total care and damage control for femoral shaft fracture in severely injured patients. J Trauma. 2005;58:446–52. discussion 452–4. [PubMed]
22. Pittet D, Rangel-Frausto S, Li N, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995;21:302–9. [PubMed]
23. Talmor H, Hydo L, Baree PS. Relationship of systemic inflammatory response syndrome to organ dysfunction, length of stay, and mortality in critical surgical illness: effect of intensive care unit resuscitation. Arch Surg. 1999;34:81–7. [PubMed]
24. Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response syndrome, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg.2006;93:738–744. [PubMed]
25. Rangel-Frausto S, Pittet D, Costignan M, et al. The natural history of the systemic inflammatory response syndrome. A prospective study. JAMA. 1995;273:117–23. [PubMed]
26. Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction: results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91:1659–68. [PubMed]
27. Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results form an international trial of 9461 patients. The PURSUIT Investigators Circulation. 2000;101:2557–67.
28. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation. An Intravenous nPA for Treatment of Infarcting Myocardium Early II trial substudy. Circulation. 2000;102:2031–7.[PubMed]
29. Granger CB, Goldberg RJ, Dabbous O, et al. for the Global Registry of Acute Coronary Events Investigators Predictors of in-hospital mortality in the global registry of acute coronary events.Arch Intern Med. 2003;163:2345–53. [PubMed]
30. Williams BA, Wright RS, Murphy JG, et al. A new simplified immediate prognostic risk score for patients with acute myocardial infarction. Emerg Med J. 2006;23:186–92. [PMC free article][PubMed]
31. Lowe GD, Machado SG, Krol WF, et al. White blood cell count and haematocrit as predictors of coronary recurrence after myocardial infarction. Thromb Hamost. 1985;54:700–3.
32. Burr ML, Holliday RM, Fehily AM, et al. Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART) Eur Heart J. 1992;13:166–70.[PubMed]
33. Furman MI, Becker RC, Yarzebski J, et al. Effect of elevated leukocyte count on in-hospital mortality following acute myocardial infarction. Am J Cardiol. 1996;78:945–8. [PubMed]
34. Vincent J-L. Dear SIRS, I’m sorry to say that I don’t like you [Another Point of View] Crit Care Med. 1997;25:372–4. [PubMed]
35. Siniorakis E, Arvanitakis S, Voyatzopoulos G, et al. Hemodynamic classification in acute myocardial infarction. Chest. 2000;117:1286–90. [PubMed]
36. Wu AH, Parsons L, Every NR, et al. Second national registry of Myocardial Infarction. Hospital outcome in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2) J Am Coll Cardiol. 2002;40:1389–94. [PubMed]
37. Roe MT, Chen AY, Riba AL, et al. for the CRUSADE Investigators Impact of congestive heart failure in patients with non-ST segment elevation acute coronary syndromes. Am J Cardiol.2006;97:1707–12. [PubMed]
38. Segev A, Strauss BH, Tan M, et al. for the Canadian Acute Coronary Syndrome Registries Investigators Prognostic significance of admission heart failure in patients with non-ST elevation acute coronary syndromes (from the Canadian Acute Coronary Syndrome Registries) Am J Cardiol.2006;98:470–3. [PubMed]
39. Maisel AS, Krishnaswamy P, Nowak RM, et al. for the Breathing Not Properly Multinational Study Investigators Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. [PubMed]
40. Nardi AE, Valenca AM, Nascimento I, et al. Panic disorder and hyperventilation. Arq Neuropsiquiatr. 1999;57:932–6. [PubMed]
41. Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus and pain sensation. The J of Physiol. 1992;454:359–71.
42. Campbell RW, Wallentin L, Verheugt FW, et al. Management strategies for a better outcome in unstable coronary artery disease. Clin Cardiol. 1998;21:314–22. [PubMed]
43. Marco CA, Plewa MC, Buderer N, et al. Self-reported pain scores in the emergency department: lack of association with vital signs. Acad Emerg Med. 2006;13:974–9. [PubMed]
44. Lofmark R, Nordlander R, Orinius E. The temperature course in acute myocardial infarction. Am Heart J. 1978;96:153–6. [PubMed]
45. Woodhead RL. Fever in relation to serum enzyme change in acute myocardial infarction. Am Heart J. 1974;88:813–4. [PubMed]
46. Ben-Dor I, Haim M, Rechavia E, et al. Body temperature – a marker of infarct size in the era of early reperfusion. Cardiology. 2005;103:169–73. [PubMed]
47. Risoe C, Kirkeby OJ, Grottum P, et al. Fever after acute myocardial infarction in patients treated with intravenous timolol or placebo. Br Heart J. 1987;57:28–31. [PMC free article] [PubMed]
48. Herlitz J, Bengtson A, Hjalmarson A, et al. Body temperature in acute myocardial infarction and its relation to early intervention with metoprolol. Int J Cardiol. 1988;20:65–71. [PubMed]
49. Gabriel AS, Ahnve S, Wretlind B, et al. IL-6 and IL-1 receptor antagonist in stable angina pectoris and relation of IL-6 to clinical findings in acute myocardial infarction. J Intern Med. 2000;248:61–6.[PubMed]


