| Author | Affiliation |
|---|---|
| Mark I. Langdorf, MD, MHPE | University of California, Irvine, Department of Emergency Medicine, Irvine, CA |
| Eric Wei, MD | University of Michigan, Ann Arbor, MI |
| Ali Ghobadi, MD | University of California, Irvine, Department of Emergency Medicine, Irvine, CA |
| Scott E. Rudkin, MD, MBA | University of California, Irvine, Department of Emergency Medicine, Irvine, CA |
| Shahram Lotfipour, MD, MPH | University of California, Irvine, Department of Emergency Medicine, Irvine, CA |
ABSTRACT
Introduction:
Chest pain (CP) patients in the Emergency Department (ED) present a diagnostic dilemma, with a low prevalence of coronary disease but grave consequences with misdiagnosis. A common diagnostic strategy involves ED cardiac monitoring while excluding myocardial necrosis, followed by stress testing. We sought to describe the use of stress echocardiography (echo) at our institution, to identify cardiac pathology compared with stress electrocardiography (ECG) alone.
Methods:
Retrospective cohort study of 57 urban ED Chest Pain Unit (CPU) patients from 2002–2005 with stress testing suggesting ischemia. Our main descriptive outcome was proportion and type of discordant findings between stress ECG testing and stress echo. The secondary outcome was whether stress echo results appeared to change management.
Results:
Thirty-four of 57 patients [59.7%, 95% confidence interval (CI) 46.9–72.4%] had stress echo results discordant with stress ECG results. The most common discordance was an abnormal stress ECG with a normal stress echo (n=17/57, 29.8%, CI 17.9–41.7%), followed by normal stress ECG but with reversible regional wall-motion abnormality on stress echo (n = 10/57, 17.5%, CI 7.7–27.4%). The remaining seven patients (12.3%, CI 3.8–20.8%) had non-diagnostic stress ECG due to sub-maximal effort. Stress echo showed reversible wall-motion abnormality in two, and five were normal. Twenty-five of the 34 patients (73.5%, CI 56.8–85.4%) with discordant results had a different diagnostic strategy than predicted from their stress ECG alone.
Conclusion:
The addition of echo to stress ECG testing in ED CPU patients altered diagnosis in 34/57 (59.7%, CI 46.9–72.4%) patients, and appeared to change management in 25/57 (43.9%, CI 31.8–57.6%) patients.
INTRODUCTION
Chest pain (CP) is a common presenting symptom in emergency department (ED) patients, accounting for 5.4% of all ED visits.1 These patients present a diagnostic dilemma, as the prevalence of coronary artery disease (CAD) is low but the consequence of misdiagnosis is high.2 After history, physical examination, electrocardiogram (ECG) and cardiac markers, most patients are found to be low risk for acute coronary syndrome. Many EDs use stress ECG alone to further risk-stratify this group and determine which patients need coronary angiography (CA) or admission. Although CA is considered the criterion reference in CAD diagnosis, it is invasive and expensive.3
According to American Heart Association/American College of Cardiology guidelines, exercise ECG is the first test for evaluation of known or suspected CAD. Patients must reach greater than 85% of their maximum heart rate on a treadmill or exercise bicycle for optimal stress.4 Stress ECG is considered positive with reversible, regional ST changes suggesting ischemia, but it has demonstrated sensitivity and specificity of 68% and 77%, respectively.5 When applied to a CPU population with low prevalence of disease, stress ECG yields many false-positives.
The addition of echocardiograph (echo) to stress ECG testing may reveal cardiac pathology and potentially better inform the decision to perform CA. Stress echo shows left ventricular systolic and diastolic dysfunction, valvular problems, infarction and stress-induced ischemia.4 It also discovers other cardiovascular diagnoses, such as pericardial effusion or aortic dissection. Reversible regional wall motion abnormalities indicate myocardial ischemia and last up to two minutes or until heart rate drops from maximal. The sensitivity and specificity of stress echo for CAD improves modestly to 79.1% and 87.1%, respectively, over stress ECG testing alone.6
Previous studies have compared stress ECG alone with the combination of stress ECG and echo, 7, 8but no study has compared the two in ED patients. We describe the use of stress echo, at our institution, to identify cardiac pathology compared with stress ECG, and its apparent effect on patient management.
METHODS
We conducted a retrospective cohort study of CP patients who also had an abnormal stress test result, either ECG stress test or stress echo and admitted to the CPU between January 1, 2002 and December 31, 2005. In the judgment of the attending emergency physician (EP), these patients’ presentations warranted diagnostic testing for CAD (initial and six-hour ECG, initial chest radiography, and initial and six-hour creatine phosphokinase-MB fraction and Troponin I) but were all negative. We performed the study in a 35-bed tertiary care ED, with a census of 38,000 patients per year, supporting a postgraduate year 1–3 emergency medicine residency. The CPU protocol ended with a stress echo in all cases. Patients had a resting echo, followed immediately with stress by treadmill or, for those unable to walk or run, with intravenous dobutamine. We considered treadmill and dobutamine equivalent stressors. Finally, the echo was repeated immediately with rapid heart rate to assess tachycardia-induced regional wall motion. Nuclear studies were not part of the CPU protocol at our institution.
We included all patients with positive stress tests of either type during calendar years 2002–2005, drawn from the cardiology stress test database (n=57). The cardiac stress lab personnel searched the database for either abnormal stress test component. A positive stress ECG was defined as a ≥1 mm of horizontal or downsloping ST-segment depression or elevation for at least 60 to 80 milliseconds after the end of the QRS complex.9 A positive stress echo was defined as normal resting wall motion but reversible hypokinesis or akinesis after exercise or dobutamine.10 We did not include any patients with nuclear imaging as CPU patients all undergo stress echo testing, nor exclude any patients with either a positive stress ECG or positive stress echo.
Patients were aged 30–76 (mean = 54). Twenty-seven were female (47.3%). Ethnicity was 59.6% Caucasian (n = 34), 17.5% Hispanic (n = 10), 10.5% Asian (n = 6), 8.8% African-American (n = 5), and 3.5% other (n = 2).
A single investigator (EKW, a fourth-year medical student with research experience) abstracted ED and hospital charts and computerized ECG and stress echo repots directly into an Excel spreadsheet (Microsoft Corporation, Redmond, WA) with standardized data elements. We recorded patient demographics, disposition, stress ECG and stress echo findings. We searched computerized records for CA reports after stress testing on all patients through 2007; if absent, we presumed they were not done at our institution. We included data from only one CP evaluation per patient. We did not have access to stress test or CA results outside our institution.
A senior emergency medicine resident assisted the medical student in interpreting and categorizing the stress test reports based solely on the attending cardiologist’s interpretation. Non-diagnostic stress tests were those where the patient did not achieve 85% of their predicted maximum heart rate for age. As the data gathered were primarily objective, we did not need to resolve any ambiguities in the medical record.
We conformed to most elements of optimum retrospective chart review. However, the chart abstracter was not blind to the hypothesis. As there was only one data abstracter and the data were objective, we did not test intrarater agreement, nor periodically monitor the data abstracter. 11, 12
We determined concordance between stress ECG and echo and whether the stress echo results provided evidence that would customarily lead to change in clinical management. Concordance was defined as both the ECG and echo components showing ischemic changes and reversible regional wall motion abnormality in similar coronary distributions. Stress echo results that identified reversible regional wall motion abnormality in the absence of ST segment ischemic changes were deemed discordant, as was a normal or abnormal stress echo in the face of suboptimal exercise level. Change in clinical management was defined as admission to the hospital from the CPU rather than customary discharge home or performance of CA. It was presumed that CPU patients would be discharged home if the stress echo were normal. Any admission or CA following the stress echo was therefore considered a change in clinical management. The local Institutional Review Board approved the study.
RESULTS
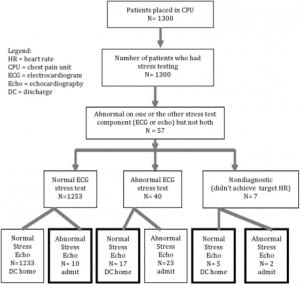
During calendar years 2002–2005, approximately 1,300 stress tests were done from the CPU, of which 57 (4.4%) had at least one component of stress testing that was abnormal, either an abnormal stress ECG, abnormal stress echo or both, and formed our study population. In 34/57 patients, the echo findings were discordant with stress ECG test results (59.7%, CI 46.9–72.4%), while in the remaining 23 CPU patients, the stress ECG and stress echo components were both abnormal.
The most common discordance was an abnormal stress ECG with a normal echo (n= 17/57, 29.8%, CI 17.9–41.7%), followed by normal stress ECG but with reversible regional wall-motion abnormality on stress echo (n= 10/57, 17.5%, CI 7.7–27.4%). The remaining seven patients (12.3%, CI 3.8–20.8%) had non-diagnostic stress ECG due to sub-maximal effort. Stress echo showed reversible wall-motion abnormality in two, and five were normal. Twenty-five of the 34 patients (73.5%, CI 56.8–85.4%) with discordant results had a different disposition than predicted from their stress ECG alone. Additionally, stress echo discovered moderate mitral regurgitation in two patients. No patient had CA based on the stress ECG component alone; all 57 patients got the echo component both before and after stress.
DISCUSSION
The addition of stress echo provided additional information about cardiac pathology in 34 of 57 patients (59.7%). It discovered reversible wall motion abnormalities in patients with normal stress ECG findings. The echo component seemed to provide sufficient reassurance for the EPs to feel comfortable discharging the patient home with no further non-invasive testing or CA.
Echo also found valvular pathology that cannot be assessed with ECG.
Two previous studies of non-ED patients made similar comparisons between stress echo and stress ECG tests.7, 8 These studies of higher risk patients had CAD rates of 71.2% and 57.3%, respectively, far higher than our ED CPU population. Salustri et al. found a lower rate of discordance between the two tests (n= 7/35, 20%) than we did (59.7%), while Severi’s published data preclude calculation of a discordance rate. However, at least 16.1% of his patients must have had discordance, while the true rate was likely much higher. Seven of our 34 discordant findings (20.6%) were equivocal stress ECG tests limited by exercise tolerance. Salustri reported no patients with equivocal stress ECG tests, while Severi reported 7.0%, both unrealistically low in our experience. Whether we consider all of our equivocal stress ECG tests as positives or negatives, our discordant rate is largely unchanged, 58.2% and 55.7%, respectively. Neither of these studies commented on additional anatomical findings with stress echo, while we found two patients with valvular disease.
Adding echo to standard ECG stress testing more than doubles the charges at our institution, from $1,103 to $2,299, including professional and technical fees. Despite this, stress echo is supported in the literature as cost-effective.3 After studying 429 patients in 1994, Severi postulated that, while stress echo outperformed stress ECG as a diagnostic test, the latter would still remain first line for screening due to lower cost.7 However, a 2008 economic analysis of strategies to diagnose CAD, including non-invasive ECG, echo, and nuclear imaging, as well as CA, found pharmacological stress echo to be the most cost-effective. Exercise stress echo was found to be 1.5 times the cost of pharmacological stress echo, while exercise stress ECG was 3.5 times higher, due to false-positives requiring CA. The same study found the cost of a primary CA strategy after history, physical, resting ECG, and cardiac markers, to be 56.3 times higher than pharmacological stress echo.13
Lewandowski, et al. reported an economic-analysis of diagnostic screening for CAD in 551 patients, beginning with baseline risk. CPU patients, who are already deemed low risk (<10% prevalence of CAD), can be further risk-stratified by gender, since at any age CAD is less common in women. Therefore, this approach recommends stress ECG in males (94% sensitivity, 27% specificity), but stress echo in females (79% sensitivity, 71% specificity) increases specificity and avoids pursuit of false-positive results.3 This differential approach by gender increases the specificity in women from 16% with stress ECG to 71% with stress echo, without sacrificing sensitivity (91% vs. 79% respectively, p = NS)
In our study, 25/34 patients with discordant stress test results had a different clinical course than predicted from stress ECG results alone. We presumed that 17 positive stress ECG tests would have required CA, but 16 did not when the echo component was negative. Five of seven with non-diagnostic ECG stress tests also would have customarily had CA to clarify pathology, but echo changed their management, as one had CA after positive stress echo, while four did not when the stress echo was normal. Of 10 more with normal stress ECG and abnormal stress echoes, four had CA. The other six should have had CA but did not at our institution. Therefore, routine stress echo in addition to stress ECG testing is supported by the bulk of previous literature, and is used at our institution to guide patient management. All CA for our patients was performed during the hospitalization following the abnormal stress echo test.
The addition of echo to stress ECG identified cardiac pathology in CPU patients and may have been used to avoid CA and hospital admission in our institution. This strategy appears to be cost-effective. However, a prospective study that compares both test components to a criterion reference of CA with clinical outcome is required to validate this approach.
LIMITATIONS
Our study had major limitations. As a retrospective review, the data in the computerized medical record was incomplete. Many of our patients did not undergo CA, even if the non-invasive testing indicated it. This, in turn, precluded determination of the test characteristics of stress testing for CAD. In addition, we could not determine the treating physicians’ pre-test treatment plans. Hence, assertions that the stress echo changed management are speculative and based on our view of customary practice.
By searching the cardiology database to identify patients with abnormal stress test results, it is possible that we omitted some patients who should have been included. The Director of Cardiac Stress Testing laboratory is facile with the database, but there has been no validation of the search strategy to assure that it captures all appropriate patients.
While some exercise ECG stress tests were indeterminate due to failure to achieve the target heart rate, the same limitation would apply to the echo portion of these patients’ tests, limiting the sensitivity to identify acute coronary syndrome at maximal load. That our cardiologists felt comfortable calling a stress echo negative short of the target heart rate has not been validated. Therefore, the disposition of five patients in the study with non-diagnostic stress ECGs appears unsupported by non-invasive testing.
As with all non-invasive testing to risk stratify CP patients, the approach in our institution leads to discharge of some patients with coronary disease. This is inherent in the limited sensitivity of diagnostic testing, whether for cardiac problems or otherwise. Hence, it is prudent to advise all discharged patients of the warning signs to return to the ED immediately and not to portray the diagnostic workup as “negative,” but rather “very low risk.”
The cardiologists reading stress echo tests were subject to incorporation bias, as they were aware of results of the stress ECG tests. We assumed that reversible regional wall motion abnormality on stress echo represented acute ischemia and suggested CAD. However, the echo findings may have been incidental, and reflected asymptomatic or chronic CAD.
Our study was too small to address which type of patient might benefit from the stress echo versus stress ECG alone. We did not, for example, stratify our patients by age, sex or beta-blocker use. This paper describes customary practice at one institution and should not be generalized.
CONCLUSION
In 59.7% of ED CPU patients in our institution, we found that stress echo testing was used to justify either patient discharge home or the need for admission or CA and to identify valvular pathology. Furthermore, stress echo appeared to alter disposition in 74% of patients who had discordant stress ECG and stress echo results. As this is a description of one institution’s approach to risk stratification of CP patients, this report should not be construed to validate such an approach. We recommend a prospective study of a larger sample to answer the question whether the addition of echo to ECG stress testing provides accurate information and to assess the test characteristics of stress echo and stress ECG alone compared to a criterion reference of CA with clinical follow up.
Footnotes
Supervising Section Editor: David E. Slattery, MD
Submission history: Submitted July 18, 2009; Revision Received February 21, 2010; Accepted March 24, 2010
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Mark Langdorf, MD, MHPE, Department of Emergency Medicine, University of California, Irvine, 101 The City Dr., Orange, CA 92868
Email: milangdo@uci.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Burt CW, McCaig LF, Rechtsteiner EA. Advance data from vital and health statistics; no 388.Hyattsville, MD: National Center for Health Statistics; 2007. Ambulatory medical care utilization estimates for 2005.
2. Pope JH, Aufderheide TP, Ruthzer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Eng J of Med. 2000;342:1163–70.
3. Lewandowski M, Szwed H, Kowalik I. Searching for the optimal strategy for the diagnosis of stable coronary artery disease. Cost-effectiveness of the new algorithm. Cardio J. 2007;14:544–51.
4. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circul. 2004;110:e82–292.
5. Gianrossi R, Detrano R, Mulvihill D, et al. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circul. 1989;80:87–98.
6. Heijenbrok-Kal MH, Fleishmann KE, Hunink MG. Stress echocardiography, stress single-photon-emission computed tomography and electron beam computed tomography for the assessment of coronary artery disease: a meta-analysis of diagnostic performance. Am Heart J. 2007;154:415–23.[PubMed]
7. Severi S, Picano E, Michelassi C, et al. Diagnostic and prognostic value of dipyridamole echocardiography in patients with suspected coronary artery disease. Comparison with exercise electrocardiography. Circul. 1994;89:1160–73.
8. Salustri A, Fioretti PM, Pozzoli MM, et al. Dobutamine stress echocardiography: its role in the diagnosis of coronary artery disease. Euro Heart J. 1992;13:70–1.
9. Gibbons RJ, Antman EM, et al. ACC/AHA 2002 Guideline Update for Exercise Testing. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol. 2002;40:1531–40. [PubMed]
10. Goldman L, Ausiello D, et al. Cecil Med. 23rd Edition. Philadelphia, PA: Saunders Elsevier; 2007. pp. 328–329.
11. Lowenstein S. Medical record reviews in emergency medicine: the blessing and the curse. Ann Emerg Med. 2005;45:452–55. [PubMed]
12. Gilbert EH, Lowenstein R, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27:305–8. [PubMed]
13. Bedetti G, Pasanisi E, Pizzi C, et al. Economic analysis including long-term risks and costs of alternative diagnostic strategies to evaluate patients with chest pain. Cardio Ultrasound. 2008;6:21, 1–9.


