| Author | Affiliation |
|---|---|
| Karis L. Tekwani, MD | Advocate Christ Medical Center, Department of Emergency Medicine |
| Hannah F. Watts, MD | Advocate Christ Medical Center, Department of Emergency Medicine |
| Cindy W. Chan, MD | Advocate Christ Medical Center, Department of Emergency Medicine |
| Steve Nanini, MD | Advocate Christ Medical Center, Department of Emergency Medicine |
| Kathleen H. Rzechula, RN | Advocate Christ Medical Center, Department of Emergency Medicine |
| Erik B. Kulstad, MS, MD | Advocate Christ Medical Center, Department of Emergency Medicine |
ABSTRACT
Introduction:
Because of its many desirable properties, etomidate is widely used as an induction agent for endotracheal intubation. However, some have recently called into question the safety of etomidate for even single-bolus use due to its known effects on adrenal suppression. We sought to compare the in-hospital mortality between septic patients given etomidate and those given alternative induction agents for intubation.
Methods:
We performed a retrospective chart review of intubated septic patients treated in our hospital. We collected data from patients over the age of 18 with sepsis who were intubated in the pre-hospital setting, in our emergency department, or on the wards of our hospital, and calculated the in-hospital mortality of each group.
Results:
We identified 181 patients with sepsis who were intubated over the study period; 135 received etomidate and 46 received alternative agents or no induction agent. Baseline characteristics, vital signs, and laboratory values were similar between the two groups. Of the 46 patients receiving alternative agents or no agent, 18 died, yielding an unadjusted mortality of 39.1% (95% CI 25.5% to 54.6%), while of the 135 patients receiving etomidate, 63 died, for an unadjusted mortality of 46.7% (95% CI 38.1% to 55.4%), P=0.38.
Conclusion:
We found a non-statistically significant 7.6% absolute increase in mortality in patients given etomidate in our small-sized study population.
INTRODUCTION
Use of the drug etomidate for continuous sedation in mechanically ventilated patients was found to have detrimental effects on patient mortality shortly after its introduction, presumably because of its measurable adrenal suppression. The package insert now cautions against prolonged infusion, citing the “hazards of prolonged suppression of endogenous cortisol and aldosterone production” (Bedford Laboratories, Bedford, OH).1–5Several authors have recently called into question the safety of etomidate for even single-bolus use, citing its demonstrated capacity to cause relative adrenocortical insufficiency after a single bolus.6–14 Additionally, a recent retrospective analysis suggests an association of etomidate with worse outcomes in septic patients.15 Nevertheless, no rigorous, prospective, randomized study has demonstrated a clinically significant adverse outcome from either continuous or single-dose use of etomidate.
In the emergency department (ED), etomidate is widely used as an induction agent for endotracheal intubation because it allows for a rapid, smooth, hemodynamically stable intubation.16,17 The implication that etomidate may increase mortality, even with a single dose, could have widespread effects given the large number of patients who receive this medication.
We sought to determine the mortality difference between patients with sepsis given etomidate and patients with sepsis given alternative induction agents by retrospectively reviewing the outcome of intubated septic patients treated in our hospital.
METHODS
Study Design, Setting, and Selection of Participants
This was a retrospective cohort study utilizing a chart review of intubated septic patients. The study was performed at a tertiary-care suburban community hospital with an annual ED census of almost 85,000 patients. We collected data from patients over the age of 18 with sepsis who were intubated in the prehospital setting, in our ED, or on the wards of our hospital over a four-year period from December 2002 to September 2006. Intubation may have occurred at any point during the patient’s hospital stay or in the field prior to arrival in the ED. Therefore, intubation was performed by paramedics, emergency physicians, house staff physicians, or anesthesiologists. The study was approved by the hospital’s Institutional Review Board with a waiver of informed consent.
Methods of Measurement
We created a standardized abstraction form using Microsoft Excel for recording the patient’s name, age, gender, medical record number, induction agent, location and time of intubation, admit, discharge date and time, whether or not steroids were given, and whether the patient lived or died. We trained the additional abstractors in its use through individual education about location of the data in the chart. However, we were unable to blind abstractors to the study objective or to patient assignment.
We identified patients by searching the ED electronic medical record database and then confirming the diagnosis of sepsis in the inpatient electronic medical record database. We searched the terms “sepsis” and “septic” in the diagnosis field of the ED records to identify patients in the ED diagnosed as septic, and reviewed these charts to determine which patients had been intubated (either in the field or in the ED). We then searched the ED records for all patients intubated in the ED, in order to find patients who had sepsis by standard criteria but were given alternative diagnoses in the ED record (such as pneumonia, respiratory failure, hypotension, or urinary tract infection). Finally, all charts were checked against electronic hospital discharge records to ensure that sepsis was a discharge diagnosis. Patients found to have had more than one admission for sepsis were considered as independent admissions. Abstractors were given printed lists of identified patient medical record numbers from which to obtain data from our ED and inpatient records. Abstraction forms were returned to a study coordinator and entered into a master Excel spreadsheet.
We recorded into the abstraction forms times of admission, intubation, discharge, status at discharge, all intubation medications used, any use of supplemental steroids either in the ED or after admission, as well as laboratory values, vital signs, and co-morbidities. Our primary outcome was the mortality difference between patients intubated with the use of etomidate versus patients intubated using any other, or no, induction agent. We selected 10% of our charts for review and testing of interrater reliability on the variables of etomidate use, steroid use, discharge condition (dead or alive) and time of intubation.
Primary Data Analysis
Baseline demographic and clinical characteristics are described using means with 95% confidence intervals and medians with interquartile ranges (IQRs). We calculated the mortality of each of the two groups with 95% confidence intervals using the Wilson method with continuity correction (VassarStats,http://faculty.vassar.edu/lowry/VassarStats.html). We compared the unadjusted mortality between the two cohorts with a χ2 test and performed survival analysis with Kaplan-Meier curves, using the Mantel-Cox Log Rank test to compare the two groups. We considered values of P < 0.05 to be statistically significant for all analyses. Analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL).
RESULTS
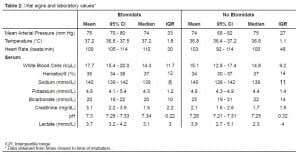
We identified 181 patients with sepsis who were intubated over the study period. Baseline characteristics were similar between the group receiving etomidate and the group receiving alternative or no induction agent, although a slightly greater percentage of patients in the non-etomidate group were immunosuppressed, on hemodialysis, or had cirrhosis (Table 1). A greater proportion of patients who did not receive etomidate were intubated after admission to the hospital when compared to the proportion of patients who did receive etomidate. Vital signs and laboratory values, shown in Table 2, were not markedly different between the two groups. Treatments rendered to patients, summarized in Table 3, were likewise not significantly different between groups, as was the percent of patients successfully extubated.
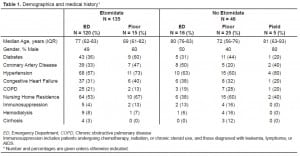
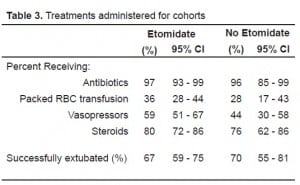
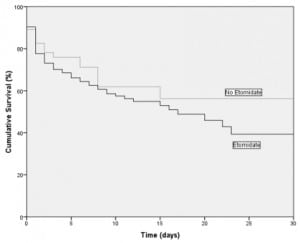
The majority of our patients, 136 (75%) were intubated in the ED, 40 (22%) were intubated after admission to the hospital, and five (3%) were intubated in the pre-hospital setting. Of the 181 patients, 135 received etomidate and 46 received alternative agents or no induction agent. Steroids were given to 108 patients (80%) in the etomidate group and 35 patients (76%) in the non-etomidate group. Of the 46 patients receiving alternative agents or no agent, 18 died, giving a mortality of 39.1% (95% CI 25.5% to 54.6%), while of the 135 patients receiving etomidate, 63 died, giving a mortality of 46.7% (95% CI 38.1% to 55.4%), P=0.38. Kaplan-Meier survival curves, shown in Figure 1, demonstrate a divergence that appears to favor the group not receiving etomidate, but the difference was not statistically significant (P=0.29). Length of ICU stay for patients receiving etomidate, 4.4 days (95% CI 3.6 to 5.1 days), was similar to patients not receiving etomidate, 5.4 days (95% CI 3.9 to 6.9 days).
All abstractors had full agreement on the use of etomidate, steroids, and discharge condition in our subset of charts reviewed for interrater reliability. Agreement for exact time of intubation was only 67%; however, the median disagreement was less than 18 minutes.
DISCUSSION
The primary motivation for the cessation of use of etomidate for continuous sedation in the ICU stems from a retrospective study of 428 severely injured trauma patients.1 In this analysis, the in-hospital mortality rate was found to have increased from 28% in those given opiates with or without benzodiazepines for sedation to over 70% after the adoption of etomidate for continuous sedation, despite similar severities of illness in the two groups of patients. After the discontinuation of the use of etomidate for continuous sedation, mortality returned to 25%. Consequently, etomidate use for continuous sedation ceased while use as an agent for intubation or procedural sedation has continued to increase. Favorable hemodynamic effects combined with a lack of immediately evident adverse effects makes etomidate particularly appealing for use in critically ill patients in the ED.
Although no studies have conclusively shown clinically significant adverse outcomes from single-dose use, a recent retrospective review of 477 patients by the Corticus Study Group found an increased odds ratio of death of 1.53 for patients sedated with etomidate.15 Additionally, a number of studies have shown adverse effects on surrogate endpoints, primarily in adrenocortical output. Reduced plasma cortisol and aldosterone levels following induction doses of etomidate have been reported to persist for up to 24 hours and appear unresponsive to adrenocorticotropic hormone stimulation.7 Patients given etomidate have shown lower measured serum cortisol compared to those given midazolam after cosyntropin stimulation tests less than 12 hours after administration and a decreased rise in cortisol from a high-dose corticotropin test 24 hours after intubation.7,18 A retrospective analysis of 152 patients with septic shock showed decreased responses to cosyntropin stimulation in patients given etomidate.9 These effects are due to blockage of 11 beta-hydroxylation within the adrenal cortex.4
With the widespread use of etomidate in the United States for induction of anesthesia prior to intubation, the potential for worsened outcomes in septic patients when using etomidate has profound implications. In our retrospective chart review, we did not find a statistically significant difference in hospital mortality between patients given etomidate and patients given alternative agents or no induction agent for intubation. The baseline demographics, vital signs, and laboratory values in the two groups suggest that the patients were similar in severity of illness and that neither group would be expected to have significantly different outcomes. Likewise, an equivalent percentage of patients in each group had been treated with steroids.
Although we did not find a statistically significant difference in outcomes between our two groups, the difference of 7.6% in absolute mortality between groups does raise a question of clinical importance. This difference perhaps supports the suggestions of others that a well-powered, prospective, randomized trial be performed to firmly establish the safety of etomidate use in patients at risk of relative adrenal insufficiency, such as those with sepsis. Given the difference in mortality seen between our patient groups, approximately 600 patients per group would be required to obtain adequate power to obtain statistical significance, using a two-sided α of 0.05 and β of 0.20.
LIMITATIONS
For this study, we relied on a chart review from a single tertiary care medical center and obtained data retrospectively, a method with well-known limitations. Although we performed an extensive search for patients, the possibility remains that we may not have found all septic patients intubated in this time period. We used an objective endpoint, discharge or death, limiting the need for observer interpretation, but many of our variables (times of intubation, types of medication used, comorbidities, vital signs) were recorded manually and are therefore subject to potential errors of transcription and omission. Although we reviewed all sections of the chart to determine which medications were used for intubation, including physician entries, nursing entries, and pharmacist entries, it remains possible that patients we classified as not having received a medication such as etomidate or steroids may in fact have received these medications, and vice-versa. We received no external funding for this study, and thus were unable to hire abstractors that could be blinded to the study purpose or to patient treatment. Although our endpoints were objective, subtle biases may have affected our results in unquantifiable or unexpected ways. Despite the inclusion of patients intubated in the pre-hospital setting (who were unlikely to have received etomidate) we found that a disproportionate number of patients received etomidate, weakening the power of our study to detect statistically significant differences in mortality. In addition, many of the patients who did not receive etomidate were intubated either in the field before ED arrival or on the hospital floors after treatment in the ED, further contributing to possible confounding.
Although we had excellent agreement between abstractors in the use of etomidate, the use of steroids, and the final discharge disposition (possibly because these items were generally documented in identical locations in each electronic medical record), we had less than perfect agreement in exact times of intubation, since in many cases documentation of procedures such as intubation were provided by more than one person, including nurses, attending physicians, and resident physicians. We limited our dependence on this measure by using the day of intubation as our start point and day of discharge or death as our endpoint. We examined many commonly used variables to evaluate differences in severity of illness between patient groups, but many charts did not have a documented Glasgow Coma Scale (GCS) score, limiting our ability to calculate scores such as a Simplified Acute Physiology Score or an Acute Physiology and Chronic Health Evaluation score. We did not attempt to estimate GCS scores from the charts we reviewed. The fact remains that the clinical appearance of the patient is often not reflected in severity of illness scores, and that unmeasured differences may exist between our cohorts that might explain the difference in mortality that we observed. Moreover, the differences in location of intubation between groups may also explain these mortality differences.
CONCLUSION
We found a non-statistically significant 7.6% absolute difference in mortality between patients given etomidate and patients given alternative or no induction agent for intubation in our study population, although the study was limited by a small sample size. Given the magnitude of this difference, further investigation into the safety of etomidate when used in patients at risk of relative adrenal insufficiency may be warranted; however, the available evidence to date does not support abandoning the use of etomidate in patients with sepsis.
Footnotes
We would like to thank Chris Blair, MS for statistical support
Supervising Section Editor: H. Bryant Nguyen, MD, MS
Submission history: Submitted December 23, 2007; Revision Received April 21, 2008; Accepted May 15, 2008.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address For Correspondence: Karis L. Tekwani, MD. Advocate Christ Medical Center, Department of Emergency Medicine, 4440 W. 95th St., Oak Lawn, IL 60453
Email: Karistekwani@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Watt I, Ledingham IM. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia. 1984;39:973–981. [PubMed]
2. Fellows IW, Bastow MD, Byrne AJ, Allison SP. Adrenocortical suppression in multiple injured patients: a complication of etomidate treatment. Br Med J (Clin Res Ed)1983;287:1835–1837.
3. Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients. Lancet. 1983;1:1270. [PubMed]
4. Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–1421.[PubMed]
5. Anonymous Etomidate Package Insert. [Accessed September 12, 2007];Bedford Laboratories. Available at:http://www.bedfordlabs.com/products/inserts/etomidate_pi.pdf.
6. Bloomfield R, Noble DW. Etomidate and fatal outcome–even a single bolus dose may be detrimental for some patients. Br J Anaesth. 2006;97:116–117. [PubMed]
7. Malerba G, Romano-Girard F, Cravoisy A, Dousset B, Nace L, Levy B, Bollaert PE. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intensive Care Med. 2005;31:388–392. [PubMed]
8. Jackson WL., Jr Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal. Chest. 2005;127:1031–1038. [PubMed]
9. Mohammad Z, Afessa B, Finkielman JD. The incidence of relative adrenal insufficiency in patients with septic shock after the administration of etomidate. Crit Care.2006;10:R105. [PMC free article] [PubMed]
10. Morris C, McAllister C. Etomidate for emergency anaesthesia; mad, bad and dangerous to know? . Anaesthesia. 2005;60:737–740. [PubMed]
11. Schulz-Stubner S. Sedation in traumatic brain injury: avoid etomidate. Crit Care Med. 2005;33:2723. Author reply: 2723. [PubMed]
12. Bloomfield R, Noble DW. Etomidate, pharmacological adrenalectomy and the critically ill: a matter of vital importance. Crit Care. 2006;10:161. [PMC free article][PubMed]
13. Zed P, Mabasa V, Slavik R, Abu-Laban R. Etomidate for rapid sequence intubation in the emergency department: is adrenal suppression a concern? . Can J Emerg Med.2006;8:347–350.
14. McConachie I. Etomidate controversies in emergency medicine. Ann Emerg Med.2007;50:200–201. [PubMed]
15. Lipiner-Friedman D, Sprung CL, Laterre PF, et al. Adrenal function in sepsis: the retrospective corticus cohort study. Crit Care Med. 2007;35:1012–1018. [PubMed]
16. Sakles JC, Laurin EG, Rantapaa AA, Panacek EA. Airway management in the emergency department: a one-year study of 610 tracheal intubations. Ann Emerg Med.1998;31:325–332. [PubMed]
17. Zed PJ, Abu-Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Acad Emerg Med. 2006;13:378–383. [PubMed]
18. Schenarts CL, Burton JH, Riker RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8:1–7. [PubMed]


