| Author | Affiliation |
|---|---|
| Robert A Aertker, BS | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| Colin M Barker, MD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas |
| H. Vernon Anderson, MD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| Ali E Denktas, MD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| Gregory M Giesler, MD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| Vinay R Julapalli, MD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| John F Ledoux, MD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| David E Persse, MD | Baylor College of Medicine, Department of Surgery, Houston, Texas; Houston Fire Department, Houston, Texas |
| Stefano Sdringola, MD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| Mary T Vooletich, RN, BSN | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
| James J McCarthy, MD | University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas University of Texas at Houston Medical School, Department of Emergency Medicine, Houston, Texas |
| Richard W Smalling, MD, PhD | University of Texas at Houston Medical School, Department of Internal Medicine–Division of Cardiology, Houston, Texas University of Texas at Houston Medical School, Memorial Hermann Heart and Vascular Institute, Houston, Texas |
ABSTRACT
Introduction:
Acute anterior myocardial infarctions caused by proximal left anterior descending (LAD) artery occlusions are associated with a higher morbidity and mortality. Early identification of high-risk patients via the 12-lead electrocardiogram (ECG) could assist physicians and emergency response teams in providing early and aggressive care for patients with anterior ST-elevation myocardial infarctions (STEMI). Approximately 25% of US hospitals have primary percutaneous coronary intervention (PCI) capability for the treatment of acute myocardial infarctions. Given the paucity of hospitals capable of PCI, early identification of more severe myocardial infarction may prompt emergency medical service routing of these patients to PCI-capable hospitals. We sought to determine if the 12 lead ECG is capable of predicting proximal LAD artery occlusions.
Methods:
In a retrospective, post-hoc analysis of the Pre-Hospital Administration of Thrombolytic Therapy with Urgent Culprit Artery Revascularization pilot trial, we compared the ECG findings of proximal and nonproximal LAD occlusions for patients who had undergone an ECG within 180 minutes of symptom onset.
Results:
In this study, 72 patients had anterior STEMIs, with ECGs performed within 180 minutes of symptom onset. In patients who had undergone ECGs within 60 minutes (n = 35), the mean sum of ST elevation (STE) in leads V1 through V6 plus ST depression (STD) in leads II, III, and aVF was 19.2 mm for proximal LAD occlusions and 11.7 mm for nonproximal LAD occlusions (P = 0.007). A sum STE in V1 through V6 plus STD in II, III, and aVF of at least 17.5 mm had a sensitivity of 52.3%, specificity of 92.9%, positive predictive value of 91.7%, and negative predictive value of 56.5% for proximal LAD occlusions. When the ECG was performed more than 60 minutes after symptom onset (n = 37), there was no significant difference in ST-segment deviation between the 2 groups.
Conclusion:
The sum STE (V1-V6) and STD (II, III, aVF) on a 12-lead ECG can be used to predict proximal LAD occlusions if performed within the first hour of symptom onset. This should be considered a high-risk finding and may prompt prehospital direction of such patients to PCI-capable hospitals.
INTRODUCTION
Acute ST-elevation myocardial infarctions (STEMI) due to occlusion of the left anterior descending (LAD) artery have a poorer prognosis than right coronary artery and left circumflex artery occlusions.1–5 One study showed that the ejection fractions for proximal LAD artery, left circumflex artery, and right coronary artery occlusions 36 hours after the event were 37%, 47%, and 50% respectively. The 3-year mortality rates for this same group were 10%, 6%, and 4%.2 Furthermore, proximal LAD occlusions are associated with a higher 30-day mortality and 3-year mortality than distal LAD occlusions in acute anterior myocardial infarctions.2,6,7 Identification of this highest-risk subgroup with the 12-lead electrocardiogram (ECG) will allow healthcare providers to rapidly assess the potential severity of the STEMI.
During an STEMI, the natural progression of ECG findings is as follows: (1) hyperacute T waves, (2) ST-segment elevation, (3) pathologic Q waves, and (4) T-wave inversion with ST-segment resolution.8,9 Extensive anterior myocardial infarctions have a steady decrease in sum ST elevation (STE) over the first 3 hours, whereas antero-septal myocardial infarctions have a fairly constant sum STE over the first 3 hours.8 A study using dogs as a model for myocardial infarctions found that maximum STE occurred within 5 to 10 minutes of complete occlusion of the left circumflex artery.10
We sought to determine if there is a relationship between the magnitude and extent of the initial ST deviation (sum of ST elevation in the precordial leads and ST depression in the inferior leads) and the location of the occlusion in the LAD artery during the early phases of an acute anterior STEMI.
METHODS
This report is a retrospective substudy of the Pre-Hospital Administration of Thrombolytic Therapy with Urgent Culprit Artery Revascularization (PATCAR) pilot trial, which enrolled a total of 338 patients with suspected acute STEMI. We analyzed the ECG findings of 72 patients with acute anterior myocardial infarctions who had undergone an ECG within 180 minutes of symptom onset.
In the PATCAR pilot trial,11 patients were randomly assigned to receive a full dose of prehospital fibrinolytics, followed by care in the critical care unit and catheterization before discharge (group A) versus receiving a half dose of prehospital Reteplase, followed by urgent percutaneous coronary intervention (group B). Groups C and D patients were ineligible for randomization and were treated with primary PCI. We included patients from all groups in this study.
The inclusion criteria for the PATCAR trial were as follows: (1) 18 years of age and older; (2) within 6 hours of symptom onset; (3) STE of 0.1 mV or greater in 2 or more contiguous limb leads or STE of 0.2 mV or greater in 2 or more contiguous precordial leads; (4) ischemic discomfort lasting more than 30 minutes. Exclusion criteria included contraindication to thrombolytic therapy or cardiac arrest requiring more than 20 minutes of cardiopulmonary resuscitation. In addition, patients were excluded from this substudy if they had had left bundle branch block.
The initial prehospital ECG performed by the paramedics was used in our analysis; therefore, the patient’s treatment group had no effect on the ECG that was used in this study. The time of symptom onset was determined by the patient’s history, which was given to the paramedics and recorded in the fire department patient care records.
The ECG reader was blinded to the angiographic results. ST elevation was measured manually 0.02 seconds after the J point. Measurements were made to the nearest 0.5 mm (0.05 mV). The TP segment was used as the isoelectric line. The STE in leads V1 through V6 were measured individually and then summed. ST depression was measured in the same manner in leads II, III, and aVF.
The patients were then categorized by the location of the occlusion on angiography. Occlusions proximal to the first septal perforator were classified as the proximal LAD group. Occlusions distal to the first septal perforator were classified as the nonproximal LAD group. The data from these 2 groups were then analyzed according to the duration of ischemic time. The interpretation of data from prior studies, which investigated the evolution of ECG changes during acute myocardial infarctions, informed our decision to divide the groups into patients who had undergone ECGs within 60 minutes of symptom onset and those who had undergone ECGs within 61 to 180 minutes from symptom onset.
A t test was used to assess differences between groups. Differences with P < 0.05 were considered statistically significant.
RESULTS
The baseline characteristics of the proximal and nonproximal patient groups who had undergone ECGs within 180 minutes of symptom onset are listed in Table 1. The systolic blood pressure of the nonproximal LAD group was significantly greater than that of the proximal LAD group. Otherwise, all other baseline characteristics were not significantly different.

Table 2 provides a summary of the STE (V1-V6) plus the ST depression (II, III, and aVF) for the proximal and nonproximal LAD groups. A significant difference (P = 0.007) is seen within the first 60 minutes of symptom onset. A significant difference in sum STE and ST depression does not exist when comparing all data within 180 minutes of symptom onset or when comparing ECGs performed within 61 to180 minutes from symptom onset.

When using a sum STE (V1-V6) plus the sum ST depression (II, III, and aVF) of at least 17.5 mm to predict proximal LAD occlusions within the first hour of symptom onset, we found a sensitivity of 52.3%, specificity of 92.9%, positive predictive value of 91.7%, and negative predictive value of 56.5%, as summarized in Table 3.

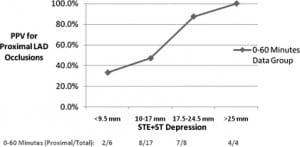
The Figure shows a comparison of the positive predictive value, using sum STE plus ST depression measurements to differentiate proximal and nonproximal LAD occlusions. As seen in this figure, the positive predictive value (PPV) increases as STE plus ST depression values increase in the 0-to-60-minute-from-symptom-onset data group.
DISCUSSION
Previous studies have shown that maximum sum STE is typically reached within the first hour of symptom onset and then gradually declines during the course of an acute anterior myocardial infarction.8–15 In our study, we were able to demonstrate that sum STE (V1-V6) plus ST depression (II, III, and aVF) is significantly different between proximal and nonproximal LAD occlusions for ECGs performed within 60 minutes of symptom onset.
Our data indicate that proximal LAD occlusions have a larger sum STE plus ST depression (mean = 19.2 ± 9.0 mm) in the first hour, which then rapidly decreases over the next 2 hours. This is similar to what has been demonstrated in other studies charting sum STE during the course of an acute myocardial infarction.8,13,14 Our data showed that nonproximal LAD occlusions have an initially smaller sum STE plus ST depression (mean = 11.7 ± 4.8 mm) within the first hour of symptom onset. The mean sum STE plus ST depression does not decrease over the first 3 hours as seen in the proximal LAD occlusions. Klainman et al8 found similar results when they evaluated anterior extensive myocardial infarctions and antero-septal myocardial infarctions. Their data show a precipitous decrease in sum STE over the first 3 hours in anterior extensive myocardial infarctions and a relatively constant sum STE in antero-septal myocardial infarctions over the same time period. Capone et al15 also had similar findings while mapping ST elevation over a 1-hour time span during induced myocardial infarctions in pigs.
It appears that the peak value in sum STE plus ST depression in proximal LAD occlusions is sustained for a shorter duration than that in nonproximal LAD occlusions. This study provides evidence that high STE plus ST depression (≥ 17.5 mm) values within the first hour of symptom onset are high-risk findings and indicate that a large area of myocardium is in jeopardy. Our criteria can potentially assist physicians in risk stratification of patients.
Previous studies have proposed various methods for identifying proximal LAD occlusions.16–18Engelen et al16 proposed a number of markers for differentiating proximal LAD occlusions from nonproximal LAD occlusions, but they have limited utility owing to their poor sensitivities. Furthermore, they only included patients with 2.0 mm or greater in V2 and V3 and excluded patients with left ventricular hypertrophy, ECG signs of a previous myocardial infarction, or previous cardiac surgery. The criteria to identify proximal LAD occlusions, proposed by Eskola et al,17 have good sensitivities (82%, 87%, and 94%) and positive predictive values (84%, 85%, and 85%). However, they have only moderate specificities (50%, 50%, and 49%). The authors also excluded patients who had already developed Q waves or inverted T waves. Arbane and Goy18 used ST-segment changes in lead aVL to identify LAD occlusions proximal to the first diagonal branch. The positive predictive values for their 2 criteria were 65% and 57%.
We did not exclude patients with multivessel disease, ECG signs of a previous myocardial infarction, Q waves, or inverted T waves, as other studies have done. We did not exclude patients with prior cardiac surgery, unless it was performed within the previous 4 weeks. Other studies excluded any patient with a history of prior cardiac surgery, regardless of when it was performed. Our assessment criteria are generalizable to many patients presenting with acute anterior myocardial infarctions.
Studies have shown that ischemic times of 20 to 40 minutes result in necrosis of at least small portions of the jeopardized myocardium.10,19 It is possible that this is reflected in the sum STE plus ST depression. The sum STE plus ST depression may rise sharply within the first few minutes of occlusion and then decrease as some cells shift from the injury stage to the necrosis stage. The surrounding ischemic penumbra then gradually becomes necrotic, but at a slower rate than in the initial stages of an acute myocardial infarction. Higher mean STE plus ST depression values in proximal LAD occlusions within the first hour of symptom onset may reflect the fact that a larger area of myocardium is in the injury stage and at risk of necrosis. Because proximal LAD occlusions disrupt a larger volume of blood flow, less collateral circulation is available to sustain the at-risk area when compared to nonproximal LAD occlusions. The injury zone of nonproximal LAD occlusions may have a longer window of opportunity to restore blood flow and avoid necrosis. The rapid decrease in sum STE seen in proximal LAD occlusions, compared to the stable sum STE seen in nonproximal LAD occlusions, may reflect this.
Recent advances in expediting reperfusion therapy for patients with STEMI include the use of the prehospital ECG to activate cardiac catheterization laboratories before the patient’s arrival. Pedersen et al20 demonstrated that the utilization of field triage for patients with STEMI reduces door-to-balloon time and all-cause mortality. According to American Heart Association data,21approximately 25% of hospitals have the capability of performing primary PCI for patients with STEMI. It is unknown what percentage of these hospitals can activate cardiac catheterization laboratories with the use of prehospital ECGs for field triage. We hope that our ECG criteria can be used in the future to augment field triage of patients with STEMI to further reduce mortality in this highest-risk subgroup.
While data are not available to compare the outcomes of different reperfusion strategies for proximal and nonproximal LAD occlusions, we think this is an area of opportunity to further study. The initial results from the Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) trial showed no benefit for the use of facilitated PCI compared to primary PCI.22 However, these initial results did show a modest trend, while not statistically significant, favoring the use of facilitated PCI in “high-risk” patients. High-risk patients were defined as having anterior infarction, ranking in Killip class above 1 upon arrival, older than age 70, or having heart rate greater than 100 beats per minute. The results also demonstrated that the use of facilitated PCI, compared to primary PCI, is associated with a statistically significant increase in the number of patients with 70% or more ST-segment resolution within 90 minutes and a TIMI grade flow of 3 before PCI. A more recent retrospective study from the FINESSE trial documented a statistically significant decrease in 1-year mortality rates for patients receiving facilitated PCI who were high risk, with a modified TIMI risk score of 3 or greater, and who presented to a spoke hospital without PCI capability within 4 hours of symptom onset.23 Additionally, long door-to-balloon times have been shown to have a greater impact on survival in these high-risk patients compared to low-risk patients, for those who presented to the hospital within 3 hours of symptom onset.24 Thus, reperfusion strategies and time-to-reperfusion may have differing outcomes, based on the location of occlusion. It remains to be seen whether or not this is applicable to proximal versus nonproximal LAD occlusions. However, given these data, it appears that facilitated PCI may have a role in the treatment of high-risk patients with STEMI who present within a few hours of symptom onset, particularly if they present to a hospital without PCI capability. Our criteria for identifying proximal LAD occlusions could be used in the future to direct reperfusion strategies once this subject has been further investigated.
LIMITATIONS
There are some limitations to this study. This is a retrospective analysis of data already accumulated during the PATCAR pilot trial. However, because the ECG reader was blinded to the angiographic findings, the post-hoc nature of the study should not have a significant impact on the data. Owing to the moderate sample size (n = 72), we should further investigate this general principle in a prospective study to confirm its validity and to refine the test criteria. Only LAD occlusions were included in this study. Patients with anomalous coronary circulation may have extensive anterior infarctions that do not involve the LAD artery as the culprit lesion. Also, given that the proximal LAD patient group had had a higher number of prior myocardial infarctions than the nonproximal LAD patient group, these patients may have potentially developed a left ventricular aneurysm after initial myocardial infarction, resulting in persistent ST-segment elevation, and thus confounding our measurements. Only patients with STEMI were included in the PATCAR pilot trial. Patients with pericarditis, which results in ST elevation, were not included in this study. This would affect the precision and accuracy of our test criteria, if used in real clinical scenarios.
CONCLUSION
This study provides a useful guideline for identifying proximal LAD occlusions, based on the electrocardiographic findings within the first hour of symptom onset. Although our criteria (STE + ST depression ≥ 17.5 mm) has only fair sensitivity (52.3%), the high PPV (91.7%) and specificity (92.9%) should allow healthcare providers to reliably identify high-risk patients with a quick and noninvasive test. The sum of STE and ST depression within the first hour of symptom onset is useful in assessing the location of occlusion in acute anterior myocardial infarctions. High values in STE plus ST depression correlate with proximal LAD occlusions within the first hour of symptom onset. Given the paucity of hospitals capable of PCI, early identification of more severe infarction may prompt emergency medical service routing of these patients to PCI-capable hospitals. Prehospital ECGs performed very early in the STEMI course correlate best with high-risk angiographic findings, consistent with the most potential benefit from reperfusion.
Footnotes
Grant support was provided in part by Centocor, Lilly, SCIOS, PDL BioPharma, Medtronic-PhysioControl, and Sanofi-Aventis.
Supervising Section Editor: Amal Mattu, MD
Submission history: Submitted August 30, 2010; Revision received January 2, 2011; Accepted February 4, 2011
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.2.2083
Address for Correspondence: Colin M. Barker, MD
Division of Cardiology, UTHSC-Houston, 6431 Fannin St, MSB 1.246, Houston, TX 77030
E-mail: colin.m.barker@uth.tmc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Thanavaro S, Kleiger R, Province M, et al. Effect of infarct location on the in-hospital prognosis of patients with first transmural myocardial infarction. Circulation. 1982;;66:742–747. [PubMed]
2. Elsman P, van’t Hof A, Hoorntje J, et al. Effect of coronary occlusion site on angiographic and clinical outcome in acute myocardial infarction patients treated with early coronary intervention.Am J Cardiol. 2006;;97:1137–1141. [PubMed]
3. Robinson K, Conroy R, Mulcahy R, et al. Relation of infarct site to 15 year prognosis in patients who survived for 28 days after a first myocardial infarction. Br Heart J. 1988;;60:470–473.[PMC free article] [PubMed]
4. Kennedy H, Goldberg R, Szklo M, et al. The prognosis of anterior myocardial infarction revisited: a community wide study. Clin Cardiol. 1979;;2:455–460. [PubMed]
5. Geltman E, Ehsani A, Campbell M, et al. The influence of location and extent of myocardial infarction on long-term ventricular dysrhythmia and mortality. Circulation. 1979;;60:805–814.[PubMed]
6. Karha J, Murphy S, Kirtane A, et al. Evaluation of the association of proximal coronary culprit artery lesion location with clinical outcomes in acute myocardial infarction. Am J Cardiol.2003;;92:913–918. [PubMed]
7. Harjai K, Mehta R, Stone G, et al. Does proximal location of culprit lesion confer worse prognosis in patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction. J Interv Cardiol. 2006;;19:285–294. [PubMed]
8. Klainman E, Sclarovsky S, Lewin R, et al. Natural course of electrocardiographic components and stages in the first twelve hours of acute myocardial infarction. J Electrocardiol. 1987;;20:98–109.[PubMed]
9. Goldberger AL. Clinical Electrocardiography. 6th ed. Philadelphia, PA: Mosby;; 1999.
10. Reimer K, Lowe J, Rasmussen M, et al. The wavefront phenomenon of ischemic cell death.Circulation. 1977;;56:786–794. [PubMed]
11. Smalling RW, Giesler GM, Julapalli VR, et al. Pre-hospital reduced-dose fibrinolysis coupled with urgent percutaneous coronary intervention reduces time to reperfusion and improves angiographic perfusion score compared with prehospital fibrinolysis alone or primary percutaneous coronary intervention: results of the PATCAR Pilot Trial. J Am Coll Cardiol. 2007;;50:1612–1614. [PubMed]
12. The TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) Trial: phase I findings.New Engl J Med. 1985;;312:932–936. [PubMed]
13. Essen R, Merx W, Effert S. Spontaneous course of ST segment elevation in acute anterior myocardial infarctions. Circulation. 1979;;59:105–112. [PubMed]
14. Selwyn AP, Ogunro EA, Shillingford JP. Natural history and evaluation of ST segment changes and MB CK release in acute myocardial infarction. Br Heart J. 1977;;39:988–994. [PMC free article][PubMed]
15. Capone R, Most A, Sydlik P. Precordial ST segment mapping. Chest. 1975;;67:577–582.[PubMed]
16. Engelen D, Gorgels A, Cheriex E, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol. 1999;;34:389–395. [PubMed]
17. Eskola M, Nikus K, Holmvang L, et al. Value of the 12 lead electrocardiogram to define the level of obstruction in acute anterior wall myocardial infarction: correlation to coronary angiography and clinical outcome in the DANAMI-2 Trial. Int J Cardiol. 2009;131:378–383. [PubMed]
18. Arbane M, Goy JJ. Prediction of the site of total occlusion in the left anterior descending coronary artery using admission electrocardiogram in anterior wall acute myocardial infarction. Am J Cardiol. 2000;;85:487–491. [PubMed]
19. Jennings RB, Sommers HN, Smythe GA, et al. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;;70:68–78. [PubMed]
20. Pedersen SH, Galatius S, Hansen PR, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2009;;54:2296–2302.[PubMed]
21. Jacobs AK, Antman EM, Faxon DP, et al. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation. 2007;;116:217–230. [PubMed]
22. Ellis SG, Tendera M, Belder MA, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. New Engl J Med. 2008;;358:2205–2217. [PubMed]
23. Hermann HC, Lu J, Brodie CR, et al. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv. 2009;;10:917–924.
24. Brodie BR, Hansen C, Stuckey TD, et al. Door to balloon time with primary percutaneous coronary intervention for acute myocardial infarction impacts late cardiac mortality in high risk patients and patients presenting early after the onset of symptoms. J Am Coll Cardiol.2006;;47:289–295. [PubMed]


