| Author | Affiliation |
|---|---|
| Tristan Knutson, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| David Della-Giustina, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| Eric Tomich, DO | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| Brandon Wills, DO, MS | Virginia Commonwealth University Health System, Department of Emergency Medicine, Richmond, Virginia Virginia Poison Center, Richmond, Virginia |
| Emily Luerssen, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| Penny Reynolds, PhD | Virginia Commonwealth University Health System, Department of Emergency Medicine, Richmond, Virginia |
Introduction Methods Results Discussion Conclusion
ABSTRACT
Introduction: The Masimo Radical-7 Pulse CO-Oximeter is a medical device recently approved by the US Food and Drug Administration that performs noninvasive oximetry and estimated venous or arterial hemoglobin measurements. A portable, noninvasive device that rapidly measures hemoglobin concentration could be useful in both austere and modern hospital settings. The objective of this study is to determine the degree of variation between the device’s estimated hemoglobin measurement and the actual venous hemoglobin concentration in undifferentiated emergency department (ED) patients.
Methods: We conducted a prospective, observational, cross-sectional study of adult patients presenting to the ED. The subjects consisted of a convenience sample of adult ED patients who required a complete blood count as part of their care in the ED. A simultaneous probe hemoglobin was obtained and recorded.
Results: Bias between probe and laboratory hemoglobin measurements was –0.5 (95% confidence interval, – 0.8 to –0.1) but this was not statistically significant from 0 (t0.05,124 = 0.20, P > 0.5). The limits of agreement were –4.7 and 3.8, beyond the clinically relevant standard of equivalency of ± 1 g/dL.
Conclusion: These data suggest that noninvasive hemoglobin determination is not sufficiently accurate for emergency department use.
INTRODUCTION
In most medical settings, the only way a medical practitioner can determine hemoglobin (Hb) concentration is through a percutaneous blood draw. Many clinical scenarios, especially those involving critically ill or injured patients, rely on single or serial Hb measurement determinations for clinical decision making. Even with current point-of-care testing, blood draws are invasive, potentially painful, time-consuming, resource intensive, costly, and may expose healthcare providers to blood-borne pathogens.
A portable, noninvasive device that rapidly measures Hb concentration could be useful in both austere and modern hospital settings. Patients with suspected blood loss could have real-time Hb determinations that could impact aggressiveness of care. The Masimo Radical-7 (Masimo Corporation, Irvine, California) is a medical device recently approved by the US Food and Drug Administration that performs noninvasive oximetry and estimated Hb measurements. Using a fingertip probe similar to a standard pulse oximetry sensor, the device noninvasively determines a hemoglobin level within 1 to 2 minutes without requiring any other equipment.
Several small studies conducted on humans and animals have shown a correlation between this noninvasive technology and venous Hb levels in controlled settings.1–4 Data collected by the manufacturer on a cohort of healthy volunteers, surgery patients, and adults undergoing a hemodilution protocol reported a correlation coefficient of 0.9 between venous and noninvasive Hb levels.5,6 Unfortunately, correlations are not appropriate for practical usage as they only describe a relationship without much useful clinical information.7
The purpose of our study is to further evaluate the accuracy of the Masimo Radical-7 technology by comparing its noninvasive venous hemoglobin measurements with actual venous hemoglobin levels in undifferentiated emergency department (ED) patients. This is the first study evaluating this technology in an ED population. Our main outcome is the determination of the degree of variation between these 2 methods.
METHODS
Study Design
We conducted a prospective, observational, cross-sectional study of adult patients presenting to our ED from February 2009 to January 2010. The ED is an urban Level II trauma center with an annual census of 70,000 to 75,000 visits and is the home of an emergency medicine residency that cares for a large population of young, generally healthy, active duty soldiers and their families, as well as many middle-aged and elderly military retirees. This study was approved by the hospital’s institutional review board (IRB).
Selection of Participants
The subjects consisted of a convenience sample of 127 adult ED patients who required a complete blood count (CBC) as part of their care in the ED. Patients could be enrolled by any attending or resident physician working in the ED. Patients not requiring a CBC as part of their ED evaluation were ineligible. The decision to obtain a CBC was at the discretion of the attending emergency physician. Study participants provided verbal consent after receiving a handwritten information sheet about the device and study. We excluded subjects younger than 18 years, pregnant women, prisoners, and those who lacked the mental capacity to refuse or consent to participation as directed by our IRB. Only a single Masimo probe Hb measurement and single laboratory Hb measurement were taken per subject. To distinguish between the values measured by the Masimo probe and the target laboratory values, it was estimated that a minimum of 70 to 80 patients would be required, assuming a Hb range of 6 to 16 g/dL, a constant analytic standard deviation of 1 g/dL, a single measurement for each patient, type I error of 5%, and a statistical power of 90%.
Study Materials
The study device was the Masimo Radical-7 Pulse CO-Oximeter handheld unit (version 7.7720 with rev-D sensors) and RDS-2 docking station (version 5129). The device uses an infrared fingertip sensor to measure several physiologic parameters in addition to venous Hb, including heart rate, oxygen saturation, carboxyhemoglobin, and methemoglobin values. Venous Hb results are given in 0.5 g/dL increments and are generally obtained in less than 1 minute. The stated venous Hb measurement range of the device is 0 to 25 g/dL, with highest accuracy between 8 to 17 ± 1.0 g/dL.
A major factor known to influence the accuracy of the device is the perfusion index (PI), a numerical assessment of signal quality and pulsatile strength, as well as an indirect measure of peripheral perfusion relative to a particular monitoring site. A PI greater than 1.0 signifies a more accurate venous Hb value. Placing the patient’s hand in a gravity-dependent position generally results in a higher PI and more accurate reading. Ambient factors such as decreased temperature or physiologic states that reduce blood flow to the fingertips may result in a lower PI and less accurate venous Hb readings. The presence of nail polish, dark skin color, and patient motion do not affect device performance. Ambient light can reduce device accuracy, and thus the manufacturer recommends and provides for the use of a dark plastic finger cover to reduce exposure.5
Data Collection and Processing
The noninvasive measurement device probe and dark plastic cover was applied to the patient’s gravity-dependent clean index finger, and the Hb value was recorded either before or just after the CBC was performed and before the results were known. Peripheral venous phlebotomy was performed in standard fashion. A unique patient identifier was recorded on the initial table, so that the provider could accurately match the venous hemoglobin level to the noninvasive value. All data were handwritten in the study log binder. This information was later transferred to an electronic spreadsheet.
Statistical Analysis
Equivalence was assumed to be within 1 g/dL of Hb. This threshold was chosen by the authors as a reasonable difference that would be unlikely to affect clinical decision making. To assess agreement between measurements obtained by the 2 methods, we performed a Bland-Altman analysis.8 This method allows quantification of the difference between the 2 techniques under consideration, and thus allows us to assess if the device has sufficient accuracy to allow acceptance of the device values in lieu of standard laboratory techniques. As articulated by Bland and Altman,7,8 statistical methods such as Pearson correlation or t tests are not appropriate for method comparison studies, as they do not allow evaluation of either difference or accuracy between techniques. These data are summarized by the mean, the bias, and the 95% confidence range of the differences between techniques (limits of agreement). Bias is the difference between the proposed method and the standard technique, and therefore estimated as the mean difference between Hb readings from the Masimo and the laboratory blood draw (standard). The limits of agreement were calculated as (mean difference ± 2 standard deviation). We evaluated the possibility of systematic variation over the range of measurement values (differences between the 2 techniques, changing as Hb measurements become more extreme) by calculating the rank correlation between the absolute differences and the average.8,9 All calculations were performed in SAS version 9.1 (Cary, North Carolina).
RESULTS
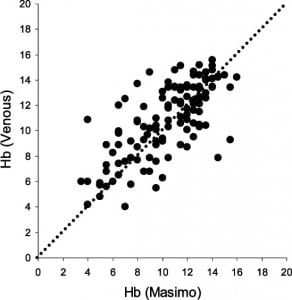
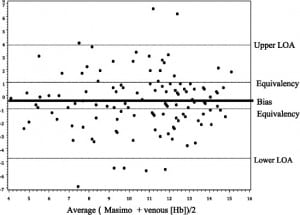
During the 11-month study period, 127 measurements were obtained from individual patients with a wide array of both medical and traumatic presentations typical of any ED of this size, paired with completed laboratory Hb determinations. Figure 1 is a scatter plot of probe Hb values versus laboratory Hb values, with a 1:1 line of equivalency. Bias was the difference between Masimo probe readings and the laboratory blood draw. Bias between probe and laboratory Hb measurements was –0.5 (95% confidence interval, –0.8 to –0.1) but this was not statistically significant from 0 (t0.05, 124 = 0.20, P > 0.5). The limits of agreement were –4.7 and 3.8, beyond the clinically relevant standard of equivalency of ± 1 g/dL. There was no systematic difference over the various hemoglobin ranges (r = 0.06, P= 0.52). There were large outliers, 2 above and 5 below the limits of agreement lines. Additionally, 23/127 (18%) were above the upper clinical equivalence boundary and 44/127 (35%) were below (see Figure 2).
DISCUSSION
Our study on a convenience sample of patients evaluated by the Masimo Radical-7 clearly showed that the device is not ready for use in clinical decision making. The limits of agreement were –4.7 and 3.8, and were beyond the prespecified, clinically acceptable range of ± 1 g/dL. These differences resulted in both overestimation and underestimation of the laboratory Hb values. This phenomenon was seen equally across all Hb ranges.
There were 8 instances in which differences of greater than 4 g/dL between the probe and laboratory measurements were noted. These outliers, combined with the patients for whom readings could not be obtained, bring into question the utility of the device for obtaining rapid, accurate results for patients who require immediate intervention.
Since this trial was completed, another iteration of software for the device has been issued. To our knowledge, this new software has not been tested clinically and its effects on the accuracy and precision of the device are unknown.
LIMITATIONS
A single laboratory Hb concentration for each patient was used as the “gold standard” and was not repeated. These measurements represent a single time point and did not evaluate accuracy of serial measurements over time. Serial probe measurements may have been able to identify if large probe variances were consistently inaccurate or if there were occasional random probe errors among a collection of accurate readings. Some of the extreme outlying values could have been due to laboratory error or inadvertent dilution rather than probe inaccuracies. Given the sheer number and standardization of laboratory blood analysis, this is unlikely. Although not recorded in our analysis, there were many instances in which a venous Hb value was obtained with a PI less than 1.0. This too could explain some of the extreme outlying values and overall performance of the device.
There was an occasional patient for whom the device did not give a reading. These patients were not included in the data analysis, nor were they tracked to determine the actual percentage of patients for whom this technology did not give any result.
Future areas of study include the evaluation of accuracy of the Masimo Radical-7 over time, using multiple measurements, as well as the impact of these measurements in clinical decision making.
CONCLUSION
In the first trial of noninvasive Hb testing done in the ED setting, the device, with the software package available to us, was not capable of providing clinically acceptable results. However, noninvasive technology is promising and should not be discounted. Further study on subsequent, planned iterations of the software and hardware should be studied for use in the ED.
Footnotes
Supervising Section Editor: James P. Killeen, MD
Submission history: Submitted February 25, 2011; Revision received August 10, 2011; Accepted September 19, 2011
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.9.6733
Address for Correspondence: Tristan Knutson, MD, Madigan Army Medical Center, Department of Emergency Medicine, MCHJ-EM,
Tacoma, WA 98431. E-mail: tristan.knutson@us.army.mil.
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none. This study was not funded or reviewed by the manufacturer. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army or the Department of Defense.
REFERENCES
1. Aldrich TK, Moosikasuwan M, Shah SD. Length-normalized pulse photoplethysmography: a noninvasive method to measure blood hemoglobin. Ann Biomed Eng. 2002;30:1291–1298. et al.[PubMed]
2. Jay GD, Racht J, McMurdy J. Point-of-care noninvasive hemoglobin determination using fiber optic reflectance spectroscopy. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2932–2935. et al. [PubMed]
3. Matsunagai K, Saigo K, Hashimoto M. Evaluation of a non-invasive hemoglobin measurement device for pre-deposited autologous blood donation [in Japanese] Rinsho Byori. 2006;54:1106–1109. et al.[PubMed]
4. McMurdy JW, Jay GD, Suner S. Noninvasive optical, electrical, and acoustic methods of total hemoglobin determination. Clin Chem. 2008;54:264–272. et al. [PubMed]
5. Masimo Rainbow SET [manufacturer data on file] Irvine, CA: Masimo Corporation; 2007.
6. Macknet MR, Kimball-Jones P, Applegate R. Continuous noninvasive measurement of hemoglobin via pulse co-oximetry [abstract] Chest. 2007;132:493. et al.
7. Bland JM, Altman DG. Measurement error and correlation coefficients. BMJ. 1996;313:41–42.[PMC free article] [PubMed]
8. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies.Statistician. 1983;32:307–317.
9. Mantha S, Roizen MF, Fleisher LA. Comparing methods of clinical measurement: reporting standards for Bland and Altman analysis. Anesth Analg. 2000;90:593–602. et al. [PubMed]


