| Author | Affiliation |
|---|---|
| Andrew C. Meltzer, MD | George Washington University School of Medicine, Department of Emergency Medicine, Washington, DC |
| Rebecca Pierce, BSN | Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland |
| Derek A.T. Cummings, PhD, MPHm, MSc | Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland |
| Jesse M. Pines, MD, MBA | George Washington University School of Medicine, Department of Emergency Medicine, Washington, DC |
| Larissa May, MD, MPH | George Washington University School of Medicine, Department of Emergency Medicine, Washington, DC |
| Meaghan A. Smith, BS | George Washington University School of Medicine, Department of Emergency Medicine, Washington, DC |
| Joseph Marcotte, BS | George Washington University School of Medicine, Department of Emergency Medicine, Washington, DC |
| Melissa L. McCarthy, ScD, MS | George Washington University School of Medicine, Department of Emergency Medicine, Washington, DC |
Introduction Methods Results Discussion Conclusion
ABSTRACT
Introduction: In emergency department (ED) patients with upper abdominal pain, management includes ruling out serious diseases and providing symptomatic relief. One of the major causes of upper abdominal pain is an ulcer caused by Helicobacter pylori (H. pylori), which can be treated and cured with antibiotics. We sought to estimate the prevalence of H. pylori infection in symptomatic patients using a convenience sample at a single urban academic ED and demonstrate the feasibility of ED-based testing.
Methods: We prospectively enrolled patients with a chief complaint of pain or discomfort in the upper abdomen for 1 year from February 2011 until February 2012 at a single academic urban ED. Enrolled subjects were tested for H. pylori using a rapid point of care 13C Urea Breath Test (UBT) [Exalenz Bioscience]. We compared patient characteristics between those who tested positive versus negative for the disease.
Results: A total of 205 patients with upper abdominal pain were tested over 12 months, and 24% (95% confidence interval: 19% to 30%) tested positive for H. pylori. Black subjects were more likely to test positive than white subjects (28% v. 6%, P < 0.001). Other factors, such as age and sex, were not different between the 2 groups.
Conclusion: In our ED, H. pylori infection was present in 1 in 4 patients with epigastric pain, and testing with a UBT was feasible. Further study is needed to determine the risk factors associated with infection, the prevalence of H. pylori in other EDs, the effect of the test on ED length of stay and the costeffectiveness of an ED-based test-and-treat strategy.
INTRODUCTION
Helicobacter pylori, H. pylori, is one of the most common worldwide human pathogens, estimated to infect the stomachs of approximately 60% of the world’s adult population.1 In the United States (U.S.), the current overall prevalence of H. pylori in adults is unknown but has been trending downward from approximately 32% in 1994.2,3 People infected with H. pylori are more likely to develop duodenal and gastric ulcers, gastric lymphoma and gastric cancer. The eradication of H. pylori is associated with ulcer healing, gastrointestinal symptom improvement and a lower likelihood of ulcer recurrence and bleeding.
Estimating prevalence is important because, in an outpatient setting with high prevalence (>10%), current gastroenterology specialty guidelines recommend a test-and-treat strategy for patients with uninvestigated dyspepsia who do not have any alarm features.4 To our knowledge, no one has investigated the prevalence of active H. pylori infection among patients who present to the emergency department (ED) with abdominal pain. The purpose of this study was to describe the feasibility of using the point-of-care 13C Urea Breath Test (UBT) to identify active H. pylori infection in patients who presented to a single, academic ED with a chief complaint of upper abdominal pain. In addition, we planned to estimate the prevalence of H. pylori as a basis for future studies and prior to implementation of a test-and-treat strategy.
METHODS
Study Design
Research assistants (RA) prospectively identified a convenience sample of adult patients with upper abdominal pain that was possibly caused by gastritis, dyspepsia or peptic ulcer disease. Eligible patients who agreed to participate signed a written consent form, answered a 1-page questionnaire and received a 13C Urea Breath Test prior to ED discharge. Subjects who tested positive for H. pylori were prescribed a treatment regimen according to the American Gastroenterology Association guidelines; for those who tested negative, treatment was left to the discretion of the primary provider. This study was approved by the university’s institutional review board.
Study Setting
The setting was a single-center, urban, academic ED with an annual volume of approximately 70,000 visits. The ED is associated with a mid-sized (371 inpatient beds) hospital with a Level 1 trauma center. The ED is staffed by board-certified emergency physicians (EP), midlevel providers and emergency medicine residents completing a 4-year residency program.
Study Population
To be eligible for the study, RAs identified patients aged 18 and older who presented to the study ED during a 1-year period beginning February 14, 2011 until February 7, 2012 with upper abdominal pain and received confirmation from the treating provider that the patient’s abdominal pain could possibly be due to gastritis, dyspepsia or peptic ulcer disease. Patients were excluded from participation if they were pregnant, currently taking antibiotics, bismuth or proton pump inhibitors (PPIs), or they were unable to walk to the testing area. We excluded patients taking antibiotics, bismuth and PPIs because these medications decrease test sensitivity.
The RAs asked all eligible subjects to sign a written consent form. The RAs were trained in clinical research through structured seminars and supervised by a senior research study coordinator working in the ED. Generally, the RAs worked weekdays between the hours of 9am – 5pm, but the coverage was not consistent throughout the study period. When a RA was working in the ED, they attempted to enroll consecutive patients.
Study Protocol
RAs administered the 13C UBT on all enrolled subjects. We used the 13C-BreathID, which is a rapid UBT that has been approved by the Food and Drug Administration for the diagnosis of H. pylori. All patients had been nil per os (NPO) for 1 hour prior to test. To perform the test, subjects breathe normally through a nasal cannula attached to the BreathID device, a machine about the size of an EKG machine or small ultrasound machine (Figure). After establishing a baseline 13CO2/12CO2 ratio, the system prompts the RA to administer a 75 mg 13C-urea tablet (tablet form 99% 13C enriched urea) and 4.5 g citric acid-based powder (4 g citric acid, 0.149 mg aspartate, orange aroma, and FD&C yellow acid (Tartrazine)) dissolved in approximately 200 mL tap water. On the basis of molecular correlation spectrometry, the BreathID continuously measures 13CO2 and 12CO2 concentrations from the patient’s breath and establishes the 13CO2/12CO2 ratio, which is displayed versus time on the screen.
High urease activity detected on exhalation is a marker of H. pylori infection with a sensitivity and specificity greater than 95%.5 The cutoff point or threshold for the BreathID has been determined to be 5 [delta] over baseline. Thus, a test result is defined as positive if the final reading is greater 5. Test sensitivity is decreased by medications that reduce organism density or urease activity; so it is recommended that bismuth and antibiotics be withheld for at least 28 days and PPIs for 7–14 days prior to the UBT. Results were obtained within 10 to 15 minutes and printed automatically. Training was provided by a manufacturer representative and consisted of a 30-minute demonstration to the principal investigator (PI) and the study coordinator. The PI and coordinator then trained the RAs to properly administer the test.
Both the subjects and the treating EPs were informed of the results after the test was completed. Interpretation of the test was performed at the point of care. Assuming no allergies to penicillin, subjects who tested positive were prescribed triple-therapy (clarithromycin 500 mg po BID, amoxicillin 1000 mg BID and omeprazole 20 mg BID for 10 days, first-line treatment per American Gastroenterological Association [AGA]) and referred to outpatient gastroenterology for followup.4 Subjects who tested negative were treated at discretion of the EP. The RAs recorded the UBT results and basic demographic information (age, gender, race, ethnicity and insurance status) using structured data collection sheets. These data were then entered into REDCap, an X electronic data capture tools.6
The primary outcome was rate of positivity for H. pylori among those enrolled. First, using chi-square test of homogeneity, we compared limited demographic and clinical characteristics of subjects with upper abdominal pain whom we enrolled in the study to all patients who presented to the study ED with a chief complaint of abdominal pain during hours when the RAs were working. Differences were considered statistically significant if the associated p-value ≤ 0.05. Second, we calculated the active H. pylori infection rate and 95% confidence interval (CI) by age, gender and race/ ethnicity. Finally, we calculated the time to disposition from a query of the electronic medical record for all groups as an objective marker of feasibility. We conducted all analyses using Statistical Analysis Software (SAS) version 9.2, Cary, North Carolina.
RESULTS
There were no significant differences between the abdominal pain patients we screened for the study during the hours when the RAs were working versus hours when the RAs were not working by demographics (age, gender or race), triage acuity or time of day or day of week (Table 1). The average age of those enrolled and tested for H. pylori was 38 years. Almost two-thirds of study subjects were female (65 %) and the majority were black (53%). Three hundred seventy-onepatients were screened for eligibility, and the most common reasons for exclusion from the study were that the patient was currently on a PPI (n = 31), the patient was currently taking antibiotics (n = 24), the patient declined the test (n = 20), or, the patient had taken bismuth or peptobismol earlier that same day (n = 18.) The remaining subjects who were screened did not have upper abdominal pain when approached.

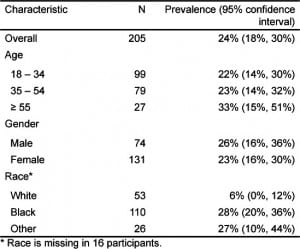
A total of 205 patients with upper abdominal pain were tested over 12 months, and 24% (95% CI: 19% to 30%) tested positive for H. pylori. H. pylori infection was significantly more prevalent among black subjects compared to whites (Table 2). Black subjects were significantly more likely to test positive than white subjects by chi-square test (28% v. 6%, P < 0.001). Other factors, such as age and sex, were not different between the 2 groups. The time to disposition appeared longer in the study group versus the general pool of abdominal pain patients. Past medical history was recorded for all enrolled subjects. Twenty-three (17.3 %) subjects reported a history of ulcer or gastritis or reflux; 4 (3%) subjects had diabetes mellitus; 7 (5.3%) had gallstones; 3 (2.3%) had liver disease; and, 4 (3%) had pancreatitis. In addition, 15 (11.3%) subjects were active smokers, 12 (9%) subjects were previous smokers and 4 (3%) reported drinking more than 5 drinks per day. Twenty-two (25.6%) subjects were currently taking PPI antacids and 22 (25.6%) reported to take NSAIDs on most days. A total of 42 (48.8%) subjects described pain that started more than 2 days prior to the ED visit. Twelve subjects received an ultrasound as part of ED evaluation, 14 received a computered tomography as part of ED evaluation, and 29 (19.3%) of subjects received intravenous narcotics as part of ED management.
DISCUSSION
This study demonstrated that approximately one-quarter of ED patients with upper abdominal pain had active H. pylori infections. Some patients infected by H. pylori may have had peptic ulcers or gastritis or non-ulcer dyspepsia, diseases in which clinical benefit has been demonstrated after eradication therapy. The test-and-treat strategy has been demonstrated to decrease morbidity and promote cost-effective care in prior studies in the outpatient setting with high prevalence.7 If prevalence is high, a similar strategy applied in the ED could benefit patients and the overall healthcare system. In our experience, the UBT was a promising test to utilize in the ED because of the rapid result, the ease of test, the tolerability of test, and the ability to change management of a common complaint. There was a small but significant increase among the study population in the percent of patients who did not receive a disposition under 2 hours. Whether the test will be feasible in other EDs that lack resources similar to our ED is unknown.
Racial and socioeconomic disparity in H. pylori infection rates have been described previously.8 If the racial disparities observed in our ED are also observed in other EDs and in follow-up studies designed to primarily explore this association, then conducting H. pylori testing in EDs that treat a predominance of non-white patients may be a useful strategy. In general, the prevalence rates that we found for whites and blacks are similar to the general population data.
We found the UBT to be easily administered by non-clinical staff and well-tolerated by ED patients. The test-and-treat strategy is recommended for outpatient settings and could be adopted in an ED with high local prevalence. Medicare reimbursement for the UBT averages $93.9 Other forms of testing for H. pylori infection, such as serum antibody tests, stool antigen test and upper endoscopy, may be less feasible in the ED. The serum antibody test does not distinguish if infection is active or resolved. The need to obtain a stool sample may make the stool antigen test more difficult during an ED visit. Finally, the upper endoscopy requires a specialist and procedural sedation.
We are currently not aware of any other U.S. EDs that routinely perform H. pylori testing. Possible reasons why testing for H. pylori is not performed in the ED include the lack of availability of the test, the idea that dyspepsia is not an emergency diagnosis, or, the concern that a patient may not receive appropriate follow-up care.10 To address follow-up access, we initially planned to follow all subjects for clinical data as outpatientsbut have not included that data due to incompleteness. In the future, we will follow patients who tested positive to determine symptom relief, H. pylori eradication rates and medication compliance.
There are potential clinical benefits to the test-and-treat strategy for ED patients with uninvestigated dyspepsia/ abdominal pain. First, eradication treatment with antibiotics can be started immediately after the initial visit in the ED.7,11 Second, patients may be spared the cost and side effects of prolonged treatment with PPIs and an invasive procedure, such as an upper endoscopy. Third, for patients with limited access to primary care and specialty care, there may be an overall reduction in incidence of long-term H. pylori-related complications, such as ulcers, gastritis or neoplasm.12 Future studies are required to address whether patients experienced symptomatic improvement after therapy and whether patients had identifiable gastrointestinal pathology such as neoplasm, gastritis or ulcer.
One potential negative result to the ED test-and–treat approach would be to provide false reassurance for a patient with pre-existing gastric cancer and to decrease likelihood that a patient would follow up with a GI specialist for diagnostic upper endoscopy. Another possible negative outcome would be to increase the risk of premature closure of diagnosis and influence a clinician to miss a different cause of the pain, such as pancreatitis.
LIMITATIONS
Our study of prevalence of H. pylori infection in the ED has 4 limitations. First, our estimate may reflect a healthier sample than the general ED population of upper abdominal pain because subjects were asked to walk to the UBT machine and not all abdominal pain patients can walk across the ED to take a test. Second, our study used a convenience sample that may introduce selection bias. We attempted to limit that source of bias by approaching sequential patients and by comparing demographics of our study sample with the general ED population. Third, we may have underestimated the H. pylori prevalence by excluding patients with active treatment for gastritis, including bismuth and patients taking PPIs. Fourth, this study occurred at a single ED, and other EDs may find a meaningfully different rate of H. pylori prevalence depending on the patient population they serve.
CONCLUSION
We have shown approximately 25% prevalence of disease in symptomatic patients and demonstrated the feasibility of using the UBT in our ED. Based on current outpatient recommendations, the test-and-treat strategy to dyspepsia should be considered in environments that have greater than 10% prevalence. Finally, given the apparent association with non-white race, this infection may represent a health disparity that should be addressed as part of a larger public health campaign.
Acknowledgments
This publication was supported by Award Number UL1RR031988 from the NIH National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The manufacturers of the Exalenz Breath Test did not provide any financial support or equipment to conduct this research.
Footnotes
Supervising Section Editor: Eric Snoey, MD
Submission history: Submitted September 20, 2012; Revision received November 20, 2012; Accepted December 10, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.12.15173
Address for Correspondence: Andrew C. Meltzer, MD, Department of Emergency Medicine, The George Washington School of
Medicine, 2120 L Street NW, Suite 450, Washington DC 20037. Email: ameltzer@mfa.gwu.edu.
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Azevedo NF, Huntington J, Goodman KJ. The epidemiology of helicobacter pylori and public health implications. Helicobacter. 2009;14(Suppl 1):1–7. [PubMed]
2. McQuillan GM, Kruszon-Moran D, Kottiri BJ. Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the united states: Data from NHANES III, 1988–1994. Am J Public Health.2004;94(11):1952–1958. et al. [PMC free article] [PubMed]
3. den Hoed CM, Vila AJ, Holster IL. Helicobacter pylori and the birth cohort effect: Evidence for stabilized colonization rates in childhood. Helicobacter. 2011;16(5):405–409. et al. [PMC free article][PubMed]
4. Chey WD, Wong BC. American college of gastroenterology guideline on the management of helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808–1825. Practice Parameters Committee of the American College of Gastroenterology. [PubMed]
5. Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of helicobacter pylori infection — a critical review. Aliment Pharmacol Ther. 2004;20(10):1001–1017. [PubMed]
6. Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. et al. [PMC free article] [PubMed]
7. Ford AC, Delaney BC, Forman D. Eradication therapy for peptic ulcer disease in helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006;(2)(2):CD003840. et al. [PubMed]
8. Epplein M, Signorello LB, Zheng W. Race, african ancestry, and helicobacter pylori infection in a low-income united states population. Cancer Epidemiol Biomarkers Prev. 2011;20(5):826–834. et al.[PMC free article] [PubMed]
9. Vakil N, Rhew D, Soll A. The cost-effectiveness of diagnostic testing strategies for helicobacter pylori.Am J Gastroenterol. 2000;95(7):1691–1698. et al. [PubMed]
10. Ufberg J. H. pylori update. Available at: http://www.emrap.org Internet. Accessed January 2011.
11. Moayyedi P, Soo S, Deeks J. Eradication of helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;2(2):CD002096. et al. [PubMed]
12. Fuccio L, Zagari RM, Eusebi LH. Meta-analysis: Can helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121–128. et al. [PubMed]


