| Author | Affiliation |
|---|---|
| Jason Cohen, DO | University of Massachusetts Medical School, Department of Emergency Medicine, Worcester, MA |
| Eric Goedecke, DO | University of Massachusetts Medical School, Department of Emergency Medicine, Worcester, MA |
| Jennifer E. Cyrkler, MD | University of Massachusetts Medical School, Department of Emergency Medicine, Worcester, MA |
| Virginia B. Mangolds, MS, FNP-C | University of Massachusetts Medical School, Department of Emergency Medicine, Worcester, MA |
| Jane Bateman, RN | University of Massachusetts Medical School, Department of Emergency Medicine, Worcester, MA |
| Karin Przyklenk, PhD | Wayne State University, Departments of Physiology & Emergency Medicine, Detroit, MI |
| Marie T. Mullen, MD | University of Massachusetts Medical School, Department of Emergency Medicine, Worcester, MA |
ABSTRACT
Introduction:
Glycemic control in the critically ill intensive care unit (ICU) patient has been shown to improve morbidity and mortality. We sought to investigate the effect of early glycemic control in critically ill emergency department (ED) patients in a small pilot trial.
Methods:
Adult non-trauma, non-pregnant ED patients presenting to a university tertiary referral center and identified as critically ill were eligible for enrollment on a convenience basis. Critical illness was determined upon assignment for ICU admission. Patients were randomized to either ED standard care or glycemic control. Glycemic control involved use of an insulin drip to maintain blood glucose levels between 80-140 mg/dL. Glycemic control continued until ED discharge. Standard patients were managed at ED attending physician discretion. We assessed severity of illness by calculation of APACHE II score. The primary endpoint was in-hospital mortality. Secondary endpoints included vasopressor requirement, hospital length of stay, and mechanical ventilation requirement.
Results:
Fifty patients were randomized, 24 to the glycemic group and 26 to the standard care cohort. Four of the 24 patients (17%) in the treatment arm did not receive insulin despite protocol requirements. While receiving insulin, three of 24 patients (13%) had an episode of hypoglycemia. By chance, the patients in the treatment group had a trend toward higher acuity by APACHE II scores. Patient mortality and morbidity were similar despite the acuity difference.
Conclusion:
There was no difference in morbidity and mortality between the two groups. The benefit of glycemic control may be subject to source of illness and to degree of glycemic control, or have no effect. Such questions bear future investigation.
INTRODUCTION
The critical care patient population is presenting more frequently to the emergency department (ED).1 Accordingly, critical care interventions are becoming a routine part of ED practice, 2 and it is a logical progression to investigate the delivery of proven critical care therapies to critically ill ED patients.
Glycemic control in critically ill patients in the intensive care unit (ICU) has been shown to attenuate both morbidity and mortality.3–5 Early therapeutic intervention in critical care patients has been shown to yield the greatest improvement in patient outcomes.6, 7 The concept of early intervention and glycemic control makes the ED an opportune arena for study. There is controversy regarding the efficacy of glycemic control. One current study challenging its practice did not have adequate power to support the discontinuation of this practice.8 The most recent study discourages intensive control, but allows for investigation of the benefit of less restrictive guidelines.9 Glycemic control remains an option in the Surviving Sepsis Campaign.10
Extrapolation of the concept of glycemic control from the ICU to the ED may have the potential for added benefit. To address this, we conducted a randomized prospective pilot study that, to our knowledge, is the first effort to investigate the effect of early management of blood glucose in critically ill ED patients.
METHODS
This was a local institutional review committee-approved study of critically ill adult patients presenting to ED at the University of Massachusetts Medical School, a tertiary referral center with approximately 75,000 adult visits per year. The University ED has a 25% inpatient admission rate, and 20% of these are admitted to the ICU. We enrolled a convenience sample between December 2004 and April 2006. Inclusion criteria were: age ≥ 18 years; critical illness (based on assignment to the ICU by a board-certified ED attending physician); and ability to obtain informed consent from the patient or surrogate. Prospective exclusion criteria were trauma-related illness; diabetic ketoacidosis or hyperosmolar hyperglycemic nonketotic coma; insulin allergy; overdoses involving hypoglycemic agents, beta-blockers or calcium channel antagonists; pregnancy, incarceration; and inability to obtain informed consent. Trauma patients have service specific protocols that would not permit inclusion in this study.
Consented patients were randomized to standard ED therapy or glycemic control. To maintain allocation concealment, patients were randomized by opening a sealed envelope determining patient’s group assignment after consent was obtained. Standard ED care was based on glucose management at the discretion of the attending physician. Patients randomized to glycemic control were managed with a standardized order sheet and nurse-driven protocol developed by the investigators specifically for the study. The goal of the protocol was to reach and maintain a blood glucose level of 80–140 mg/dL. Glycemic control patients had bedside whole blood glucose levels checked every 1–2 hours. Patients with glucose levels greater than 140 mg/dL were placed on an insulin drip (prepared off-site at a standard concentration by the hospital pharmacy), bedside glucose levels were checked every hour, and the drip rate was adjusted based on glucose levels. The standard protocol order sheet provided for drip cessation for glucose levels < 80 mg/dL. Hypoglycemia (glucose levels < 60 mg/dL) was treated with a 50 ml of 50% dextrose in water. The insulin drip was temporarily stopped when the patient left the ED for testing and was permanently discontinued at the time of discharge from the ED.
We reviewed charts from all enrolled patients. The primary endpoint of the study was in-hospital mortality. Secondary endpoints included hospital length of stay, vasopressor use, mechanical ventilator days, transfusion requirement, and renal failure requiring dialysis. Patient demographics and APACHE II (Acute Physiology and Chronic Health Evaluation) scores were recorded. Continuous variables are reported as mean ± SEM and compared between the glycemic control versus standard care groups by Student t-test. Categorical variables are presented as percentage of total and compared between groups by Fisher’s exact test.
RESULTS
We identified 66 patients as eligible for the study. Fourteen patients or their families declined, one patient was legally incompetent to consent and did not have a guardian present, and one patient was not offered treatment at the discretion of the ED physician. The remaining 50 patients were successfully consented and enrolled, with 26 assigned to receive standard therapy and 24 to glycemic control.
In the standard treatment arm, 19 of 26 patients (73%) presented with serum glucose greater than 140 mg/dL. Five of these 19 (26%) were managed by the ED with insulin therapy. Four received intravenous boluses of insulin, while one patient with sepsis was placed on an insulin drip. Insulin was initiated for these five patients in response to high glucose levels. Mean glucose for this cohort was 302 ± 127.
In the glycemic control arm, 10 of the 24 randomized patients (42%) had initial glucose values >140 mg/dL. The difference in initial glucose level between the standard and glycemic control cohort was statistically significant (p=0.04). Four patients had a glucose of >140 mg/dL and did not receive treatment per protocol at any time. These patients were included in the analysis on an intention-to-treat basis. Of the 24 patients in the glycemic control group, 16 (67%) achieved the glucose values within 80–140 mg/dL target. There was a trend toward a reduction in blood glucose levels in the 24 patients assigned to receive glycemic control versus the standard therapy (167 ± 14 versus 210 ± 22 mg/dL; p=0.11).
Four of 24 patients randomized to glycemic control (17%) experienced one episode of hypoglycemia. While three of 24 patients (13%) were actually receiving insulin at the time of the event, one of the four patients developed hypoglycemia without insulin therapy, presumably as a function of his illness. No hypoglycemic events occurred in the standard therapy group (p=0.05).
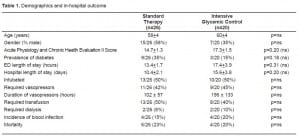
Demographics and in-hospital outcome
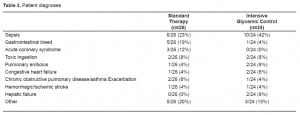
Both groups were well matched for age and gender. Despite randomization, there was a trend toward higher APACHE II scores in the treatment group as opposed to controls: mean scores of 17.2 ± 1.5 in the treatment group versus 14.7 ± 1.3 for the standard therapy group (p=0.20). Hospital length of stay trended toward a longer duration in the glycemic control group (Table 1). The requirement for intubation, vasopressors, transfusion and dialysis were similar in the two cohorts (Table 1). There was a higher percentage of patients with diabetes mellitus, based on past medical history in the standard treatment group (9/26 [35%]), as opposed to the glycemic control group (4/24 [17%]) (p=0.20). Critical care diagnoses were variable, ranging from sepsis to gastrointestinal bleed (Table 2).
DISCUSSION
The ED offers an opportunity for early intervention as it is frequently the first point of entry for the critically ill patient population.6,7 This pilot study represents, to our knowledge, the first attempt to administer early glycemic control in the ED.
Multiple logistic issues in the ED may limit the ability to deliver critical care therapies, including management of blood glucose. The onset of therapy may be delayed or deferred in instances in which patient volumes are high and nursing resources limited. In four patients enrolled in the current study, insulin per protocol was not administered despite randomization to the intensive glycemic control group. Conversely, four patients had hypoglycemia in the treatment arm. We hypothesize that these protocol violations and complications may have occurred at times of high ED volume and nursing workload.
We hypothesize that the ED environment also provided a barrier to successful glycemic goals. Insulin drip initiation or glucose level monitoring may have been delayed due to high nursing workload. Insulin drip delivery from the pharmacy may have added to treatment delay. Patients also were transferred to the ICU prior to successful completion of the glycemic protocol. Average transfer time to the ICU during the study period was 15 hours, with a range of 2 – 72 hours.
Despite these challenges, we report that 24 critically ill patients with high APACHE II scores were treated with a glycemic control protocol, and in 16 of these 24 patients the goal of successful glycemic control was achieved. Hypoglycemia did occur, but we did not measure a resultant increase in hospital mortality. We acknowledge again that the study population is too small to appreciate a significant mortality effect from hypoglycemia.
There was a trend toward higher acuity in the glycemic control group with equivalent mortality, but this trend was not statistically significant. Hypoglycemia in the treatment group presented a challenge as in other studies.8, 9 The risk of hypoglycemia in the ED environment can be of greater consequence as it may not be recognized as quickly as in a more controlled ICU. Further evaluation of glycemic control in the ED should provide for stringent precautions for hypoglycemia.
LIMITATIONS
Conclusions regarding the potential benefits of early insulin therapy initiated in the ED are precluded by the small sample size. Our study was not powered to discern a difference in mortality, hospital length of stay, vasopressor use, mechanical ventilator days, transfusion requirement, or renal failure requiring dialysis.
CONCLUSION
The NICE-SUGAR investigators have confirmed the lack of utility of intensive glycemic control as well as increased hypoglycemia risk.9 Questions regarding range of glucose control and appropriate critical care patient population remain unanswered. Larger-scale, randomized protocols should be considered in order to pursue this concept and establish whether early management of blood glucose in the ED will improve patient outcome.
Footnotes
Supervising Section Editor: Jeffrey Sankoff, MD
Submission history: Submitted February 24, 2009; Revision Received August 5, 2009; Accepted August 22, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Marie Mullen, MD, Department of Emergency Medicine, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655
E-mail: mullem01@ummhc.org
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. This study was supported in part by a Society of Academic Emergency Medicine – Geriatric Research Grant.
REFERENCES
1. Goldstein RS. Management of the critically ill patient in the emergency department: focus on safety issues. Crit Care Clin. 2005;21:81–89. viii–ix. [PubMed]
2. Rivers EP, Nguyen HB, Huang DT, Donnino MW. Critical care and emergency medicine. Curr Opin Crit Care. 2002;8:600–6. [PubMed]
3. Van den Berghe G. Dynamic neuroendocrine responses to critical illness. Front Neuroendocrinol.2002;23:370–91. [PubMed]
4. Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. [PubMed]
5. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. [PubMed]
6. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med.2006;34:1589–96. [PubMed]
7. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. [PubMed]
8. Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. [PubMed]
9. The NICE-Sugar Investigators Intensive versus Conventional Glucose Control in Critically Ill Patients. N Engl J Med. 2009;360:1283–97. [PubMed]
10. Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. [PubMed]


