| Author | Affiliation |
|---|---|
| David F. Gaieski, MD | University of Pennsylvania School of Medicine, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Byron C. Drumheller, MD | University of Pennsylvania School of Medicine, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Munish Goyal, MD | Washington Hospital Center, Department of Emergency Medicine, Washington, DC |
| Barry D. Fuchs, MD | University of Pennsylvania School of Medicine, Department of Internal Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Philadelphia, Pennsylvania |
| Frances S. Shofer, PhD | University of Pennsylvania School of Medicine, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Kara Zogby, BSN | University of Pennsylvania School of Medicine, Department of Emergency Medicine, Philadelphia, Pennsylvania |
ABSTRACT
Introduction:
Early recognition of elevated lactate levels in sepsis may hasten the detection of those patients eligible for aggressive resuscitation. Point-of-care (POC) testing is now increasingly available for use in the emergency department (ED). We examined the accuracy and time-saving effect of a handheld POC device for the measurement of fingertip and whole blood lactate as compared with reference laboratory testing in critically ill ED patients.
Methods:
A convenience sample of adult ED patients receiving serum lactate testing was prospectively enrolled at an urban, tertiary care US hospital. Consenting patients underwent fingertip POC lactate measurement with a portable device and simultaneous whole blood sampling for analysis by both the POC device and standard laboratory analyzer (“reference method”). Lactate measurements were compared by intraclass correlation (ICC) and Bland and Altman plots. Differences in time to test result were compared by paired t test.
Results:
Twenty-four patients, 19 (79%) with sepsis and 21 (88%) with lactate levels below 4 mmol/L, were included from April 2005 to May 2005. Fingertip POC and whole blood POC lactate measurements each correlated tightly with the reference method (ICC = 0.90 and ICC = 0.92, respectively). Mean time between obtaining fingertip lactate samples and whole blood reference lactate samples was 8 ± 13 minutes. Mean time between obtaining POC and reference laboratory lactate results was 65 minutes (95% confidence interval, 30–103).
Conclusion:
Fingertip POC lactate measurement is an accurate method to determine lactate levels in infected ED patients with normal or modestly elevated lactate values and significantly decreases time to test results. These findings should be verified in a larger, more critically ill, ED population.
INTRODUCTION
Sepsis is defined as the systemic inflammatory response to infection. When associated with organ dysfunction, sepsis is considered severe and is accompanied by an increased risk of mortality. Like myocardial infarction and stroke, sepsis is a time-sensitive condition amenable to early intervention. Serum lactate level obtained in the emergency department (ED) is predictive of mortality among patients with suspected infection and is used to help determine which septic patients are candidates for early, aggressive resuscitation.1–3 International consensus-based guidelines on sepsis recommend that serum lactate measurement be available with a rapid turnaround time (“within minutes”) to help identify patients with tissue hypoperfusion who are at increased risk of morbidity and mortality.4
Several obstacles exist to the rapid determination of lactate levels in the ED and the use of these results in patient care. Prolonged ED wait times, whether due to increased patient volume, protracted boarding times for admitted patients, or local hospital closures, have an impact on time to patient triage and evaluation.5 Further delays result from limitations in traditional laboratory analysis, in which test results can take hours to return and must be actively sought out by the clinician. Point-of-care (POC) devices have recently been implemented in a wide range of clinical settings, from ED triage to the intensive care unit (ICU), to hasten detection of time-sensitive disease states and expedite care.6–8 While the accuracy of whole blood POC lactate has been validated in ED and ICU patients, the performance of POC fingertip lactate measurement has been called into question.7,8 Furthermore, studies to date of ED POC lactate measurement in sepsis have not evaluated the use of a handheld device, which may allow for even more rapid determination of test results in the hectic ED environment.9
The purpose of this study was to determine the accuracy of a handheld POC device for the measurement of fingertip lactate, as compared with (1) whole blood POC and (2) whole blood laboratory testing, in critically ill ED patients. We hypothesized that both whole blood and fingertip POC measurements would closely correlate with laboratory testing and that POC testing would significantly decrease time to lactate results.
METHODS
Study Design
Prospective, observational study of a convenience sample of adult ED patients conducted from April 2005 to May 2005. The research design was preapproved by the Institutional Review Board for Human Research at the Hospital of the University of Pennsylvania. Written informed consent was obtained from all patients before enrollment.
Study Setting and Population
The study was conducted in a 700-bed urban tertiary care hospital with a 56-bed ED that provides care to approximately 55,000 adult patients annually. All patients receiving serum lactate testing ordered by the treating emergency physician were eligible for inclusion. Patients were excluded only if fingertip or whole blood venipuncture samples could not be technically obtained, which did not occur in any patients eligible for the study. No formal departmental protocol encouraging lactate measurement in patients with suspected sepsis was in place at the time of study enrollment. The purpose of examining the accuracy of the POC device was for future research examining POC lactate as a screening tool at ED triage.10
Study Protocol
Point-of-care analysis was performed with a Lactate Pro analyzer (LT-1710, Arkray Inc, Kyoto, Japan), which uses a 5-μL sample, has a detection range from 0.8 to 23.3 mmol/L, and provides results in 60 seconds. This device is Clinical Laboratory Improvement Amendments (CLIA)–approved for POC testing of lactate in the United States. For the purposes of statistical analysis, values registering less than 0.8 mmol/L, read as “Lo” on the device, were coded as 0.7 mmol/L. The device uses single-use test strips containing an enzyme-coated electrode.10,11 The machine was calibrated by one of the study investigators by using a factory-supplied calibration strip, and tested by using a check strip with a known value, every 8 hours while in use. The principal investigators (M.G., D.F.G.) received in-depth training on use of the POC device from a representative of the manufacturer and then trained the remaining study investigators, who all performed at least 2 fingertip samples on the principal investigators before enrolling subjects into the study. Documents related to CLIA approval of the Lactate Pro POC device were reviewed by the hospital’s POC testing specialists before the start of the study.
Consenting patients received a finger-prick with a disposable lancet to puncture the skin and obtain capillary blood for POC analysis. Whole blood samples (approximately 5 cc) were subsequently obtained from a venipuncture site or indwelling arterial catheter and collected in grey-top tubes (BD Vacutainer, sodium fluoride 10 mg/potassium oxalate 8 mg). Fingertip and whole blood samples were drawn as close in time as possible. A single drop of whole blood was used to measure POC lactate concentration with the Lactate Pro device. Whole blood grey-top tube samples were sent to the central hospital laboratory per institutional practice and were analyzed on a Vitros 950 analyzer (Ortho-Clinical Diagnostics, Rochester, New York), which served as the “reference method.” The Vitros 950 analyzer measures whole blood lactate level in approximately 12 minutes and was calibrated and maintained according to manufacturer standards. Whole blood reference method results were entered into the hospital electronic medical record by laboratory personnel. No messages were sent or callbacks made to the treating physicians regardless of the lactate result, since elevated lactate values were not considered critical values in our hospital laboratory at the time the study was conducted. Treating ED physicians were blinded to POC test results.
Measurements
Triage time and the time of blood sampling for lactate analysis, as well as the reason for obtaining lactate measurement, were recorded for all patients. The primary outcome measure was the accuracy of fingertip POC lactate in comparison to the reference method for lactate analysis. Secondary outcomes were the accuracy of whole blood POC lactate compared to the reference method and the time differential from fingertip POC lactate result to laboratory reference method result. Data were documented on collection forms and then entered into database software (Access 2000, Microsoft Corp, Redwood, Washington).
Data Analysis
Agreement of the POC device with the laboratory reference method was assessed by calculating the intraclass correlation coefficient (ICC), and ICC values greater than 0.9 were considered excellent agreement. To determine the variability of the POC device, as compared to the reference method, Bland and Altman plots12 were developed with mean difference and limits of agreement. Fingertip POC and whole blood POC values were each compared with the reference method. Post hoc analysis demonstrated that a sample size of 24 subjects with 2 observations per subject achieves 89% power to detect an intraclass correlation of 0.900 under the alternative hypothesis when the intraclass correlation under the null hypothesis is 0.700, using an F test with a significance level of 0.05000. The mean difference in the time between blood sampling and determination of lactate results between assays was compared by paired t test. Analyses were performed with SAS statistical software (version 9.1, SAS Institute, Cary, North Carolina).
RESULTS
Twenty-five patients consented and were enrolled in the study. One patient was withdrawn from the study because of incomplete data collection. Seventy blood samples were taken from the remaining 24 patients; 24 fingertip and 22 whole blood samples were analyzed with the POC device and 24 whole blood samples were analyzed by the reference method. Patient characteristics upon inclusion are shown in the Table. Most patients (79%) presented with sepsis (≥2 Systemic Inflammatory Response Syndrome criteria and suspected infection). Three patients (13%) had suspected infections but did not meet criteria for sepsis. Three patients (13%) were discharged to home, while the other 21 (87%) were admitted to the hospital and 9 (38%) were admitted to the intensive care unit.
Table. Demographics.
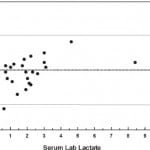
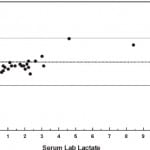
Fingertip POC lactate measurement correlated closely with the reference method, with ICC equal to 0.90 (Figure 1). The Bland and Altman plot demonstrated that fingertip POC measurements more often measured slightly higher (mean difference= −0.3 with limits of agreement between −2.4 and 1.9, Figure 1). Only 2 values fell outside of the limits of agreement (−2.4, 1.9); 1 patient with POC value equal to 3.3 mmol/L and laboratory value equal to 0.6 mmol/L and another patient with POC value equal to 7.2 mmol/L and laboratory value equal to 10 mmol/L. Whole blood POC lactate measurement correlated more closely with the reference method, with ICC equal to 0.92 (Figure 2). The Bland and Altman plot demonstrated a mean difference of 0.25, with limits of agreement between −1.2 and 1.7 (Figure 2). In this case, whole blood POC measurements more often measured lower than the reference method. Only 1 value fell outside of the limits of agreement.
Figure 1. Reference versus fingertip point-of-care (POC). Dotted line represents the mean difference between reference value and POC value. Dashed lines represent limits of agreement (95% confidence interval). FS, fingerstick.
Figure 2. Reference versus whole blood point-of-care (POC). Dotted line represents mean difference between reference value and POC value. Dashed lines represent limits of agreement (95% confidence interval). WB, whole blood.
Mean time between fingertip POC blood sampling and whole blood reference sampling was 8 ± 13 minutes. Mean time between whole blood POC sampling and whole blood reference lactate sampling was 4 ± 13 minutes. Mean time from triage to fingertip POC lactate result was 86 minutes (95% confidence interval [CI] = 13–159), while mean time from triage to whole blood reference lactate result was 151 minutes (95% CI= 73–230). Mean time to fingertip POC lactate result was shorter than whole blood reference lactate result by 65 minutes (95% CI, 30–103; P < 0.005).
DISCUSSION
The use of POC blood lactate measurement has been examined in the care of critically ill ICU and trauma patients but has only recently been studied in an ED population. Whole blood POC lactate measurement has previously been shown to correlate well with laboratory-measured whole blood lactate in the ED, which our results support.9 However, Boldt et al8 recently cautioned against the use of fingertip POC lactate because of suboptimal accuracy when compared to laboratory-analyzed arterial blood in an ICU population. Our results, however, demonstrated good agreement between fingertip POC and whole venous blood laboratory-determined lactate levels. While Boldt et al8 used a different POC device than that used in the current study, the difference may also be attributed to the patient population tested. Patients in the ICU have often received large volumes of intravenous fluid resuscitation which, in addition to continued capillary leak and decreased intravascular osmotic pressure, can lead to diffuse tissue edema.13 In contrast, critically ill patients presenting to the ED are often hypovolemic, potentially decreasing the amount of extravascular fluid that enters a fingertip blood sample. This may account for the improved accuracy noted in our study compared to prior trials.
We also found that use of a handheld POC lactate device reduced the time to obtain test results as compared to the reference method by 65 minutes. This represents a significantly greater time than the 12 minutes required by the laboratory device to display test results. Although turnaround time for laboratory blood sampling measurement is institution specific, several factors, such as time spent during physician evaluation, test ordering, or blood sampling and availability of laboratory personnel, delay test results in many hospitals. Mislabeling or misplacing samples can also delay time to test results using central laboratory testing. A handheld POC device provides an immediate result, visualized by the bedside care provider in real time, as opposed to results of standard ED POC and central laboratory testing, which must be actively sought out in the medical record by the clinician. Use of handheld, portable POC lactate measurement, either by bedside care providers, emergency medical services personnel, or as a screening tool at ED triage, could allow for immediate risk stratification of potentially critically ill patients, a strategy that has recently undergone preliminary evaluation.10,14 Combined with initial history and bedside evaluation, such results could allow for rapid administration of time-sensitive sepsis therapies, such as broad-spectrum antibiotics and aggressive fluid resuscitation.15 When the speed of obtaining lactate results provided by a POC device is combined with the risk stratification ability of an initial lactate reading for ED patients with severe sepsis,3 POC lactate measurement has significant clinical utility. Handheld fingertip lactate meter may also allow for repeated lactate levels to be more readily obtained after initial resuscitation, which could be used to determine lactate clearance. Lactate clearance has recently been shown to be equivalent to invasive central venous oxygen saturation monitoring in ED-based early goal-directed therapy of severe sepsis and septic shock.16
LIMITATIONS
This study has a number of limitations. Severe sepsis was not a strict inclusion criterion for this study; thus, our results may not be fully generalizable to all patients along the sepsis spectrum. However, most patients presented with sepsis (79%) or a clinically significant suspected infection (13%). Another limitation was that we did not standardize the location or tourniquet time for whole blood or POC sampling. By restricting blood flow to the distal portion of the limb, a tourniquet may theoretically cause increased anaerobic metabolism leading to an elevation in capillary blood lactate levels. One recent study using healthy volunteers, however, showed this assumption to be incorrect.17 Nonetheless, our results showed that POC lactate values were biased slightly higher than reference lactate results, which could be a consequence of this phenomenon. Standardization of tourniquet time and location would be ideal, but in practice is difficult in the emergency setting and in critically ill patients with potentially limited peripheral venous access. We also did not examine the precision of the POC device in this population, though prior work has validated this parameter in healthy individuals.11 Another key limitation is the small sample size. With only 24 patients, of whom only 3 had whole blood lactate levels of 4 mmol/L or greater, we can only draw limited conclusions regarding the ability of the POC device to accurately measure serum lactate levels in patients with sepsis who are eligible for early goal-directed resuscitation.1 Given that the few elevated lactate levels obtained in our study showed a slightly greater disagreement between POC and reference results, this limitation must be explicitly addressed before use of this handheld device is considered beyond an experimental setting. Formal cost-effectiveness analyses should also be conducted to examine the economic impact of bedside POC lactate testing in sepsis.
CONCLUSION
In conclusion, fingertip POC lactate measurement closely correlated with reference laboratory whole blood testing in an ED population consisting primarily of patients with sepsis and normal or modestly elevated lactate levels. Use of a handheld POC lactate device also reduced time to lactate test results. The small sample size and small number of elevated (>4 mmol/L) values limit conclusions regarding the use of this device in patients eligible for early goal-directed therapy. Further studies are needed to verify these results in a larger population, particularly patients with shock or severe global tissue hypoxia, and to test the effects of early detection of lactate levels on the prognosis and treatment of ED patients with sepsis.
Footnotes
Supervising Section Editor: Larry Raney, MD
Submission history: Submitted January 11, 2011; Revision received April 15, 2011; Accepted May 11, 2011
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.5.6706
Address for Correspondence: David F. Gaieski, MD, University of Pennsylvania School of Medicine, Department of Emergency Medicine, 34th and Spruce streets; Ground Ravdin, Philadelphia, PA 19104
E-mail: gaieskid@uphs.upenn.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. None of the researchers involved in this project have received compensation from, have intellectual property interest in, or own stock in the manufacturers of the point-of-care lactate device used to conduct the research. No manufacturer sponsorship was involved in the study. The device and cartridges used were provided by the manufacturer at no cost. Arkray, Inc, had no role in study design, data acquisition, data analysis, or writing of the manuscript. They did not review the manuscript at any point during the submission process.
REFERENCES
1. Rivers E, Nguyen B, Havstad S. et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. [PubMed]
2. Shapiro N, Howell M, Talmor D. et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528. [PubMed]
3. Mikkelsen ME, Miltiades AN, Gaieski DF. et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1677. [PubMed]
4. Dellinger RP, Levy MM, Carlet JM. et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. [PubMed]
5. Purnell L. Reducing waiting time in emergency department triage. Nurs Manage. 1995;26:64Q, 64T, 64V.
6. Degroote N, Pieper B. Blood glucose monitoring at triage. J Emerg Nurs. 1993;19:131–133.[PubMed]
7. Slomovitz B, Lavery R, Tortella B. et al. Validation of a hand-held lactate device in determination of blood lactate in critically injured patients. Crit Care Med. 1998;26:1523–1528. [PubMed]
8. Boldt J, Kumle B, Suttner S. et al. Point-of-care (POC) testing of lactate in the intensive care patient. Accuracy, reliability, and costs of different measurement systems. Acta Anaesthesiol Scand.2001;45:194–199. [PubMed]
9. Shapiro N, Fisher C, Donnino M. et al. The feasibility and accuracy of point-of-care lactate measurement in emergency department patients with suspected infection. J Emerg Med.2010;39:89–94. [PMC free article] [PubMed]
10. Moore C, Jacob S, Pinkerton R. et al. Point-of-care lactate testing predicts mortality of severe sepsis in a predominantly HIV type 1-infected patient population in Uganda. Clin Infect Dis.2008;46:215–222. [PubMed]
11. Shimojo N, Naka K, Uenoyama H. et al. Electrochemical assay system with single-use electrode strip for measuring lactate in whole blood. Clin Chem. 1993;39:2312–2314. [PubMed]
12. Bland J, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed]
13. Koomans H, Boer W. Causes of edema in the intensive care unit. Kidney Int Suppl. 1997;59:S105–S110. [PubMed]
14. Goyal M, Pines J, Drumheller B. et al. Point-of-care testing at triage decreases time to lactate level in septic patients. J Emerg Med. 2010;38:578–581. [PubMed]
15. Gaieski DF, Mikkelsen MM, Band RA. et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2009;38:1045–1053. [PubMed]
16. Jones A, Shapiro N, Trzeciak S. et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746.[PMC free article] [PubMed]
17. Jones A, Leonard M, Hernandez-Nino J. et al. Determination of the effect of in vitro time, temperature, and tourniquet use on whole blood venous point-of-care lactate concentrations. Acad Emerg Med. 2007;14:587–591. [PubMed]