| Author | Affiliation |
|---|---|
| David I. Bruner, MD | Naval Medical Center Portsmouth, Emergency Medicine Program, Portsmouth, Virginia |
| Amy M. Pritchard, DO | Naval Medical Hospital Camp Pendleton, Oceanside, California |
| Jonathan Clarke, MD | Naval Medical Center Jacksonville, Jacksonville, Florida |
Introduction
Case report
Discussion
Conclusion
ABSTRACT
While complete molar pregnancies are rare, they are wrought with a host of potential complications to include invasive gestational trophoblastic neoplasia. Persistent gestational trophoblastic disease following molar pregnancy is a potentially fatal complication that must be recognized early and treated aggressively for both immediate and long-term recovery. We present the case of a 21-year-old woman with abdominal pain and presyncope 1 month after a molar pregnancy with a subsequent uterine rupture due to invasive gestational trophoblastic neoplasm. We will discuss the complications of molar pregnancies including the risks and management of invasive, metastatic gestational trophoblastic neoplasia.
INTRODUCTION
Invasive, metastatic gestational trophoblastic disease (GTD) is very rarely seen in the emergency department (ED), but it must be recognized and treated appropriately and immediately to prevent serious complications. Gestational trophoblastic disease is a term that comprises multiple different disease processes, including hydatidiform molar pregnancy, choriocarcinoma, persistent/invasive gestational trophoblastic neoplasia (GTN), and placental site trophoblastic tumors (PSTT).1 It can present with a wide variety of symptoms depending on the site of metastasis or the extent of invasive growth, but this disease process is very rarely documented in the emergency medicine literature. We present a case of a young woman who presented 30 days after evacuation of a molar pregnancy with invasive and metastatic GTN complicated by a uterine rupture and hemoperitoneum.
CASE REPORT
A 21-year-old woman presented to the ED with a 4-day history of increasing abdominal and pelvic pain associated with nausea, generalized fatigue, and orthostatic light-headedness. She denied fever, chills, emesis, syncope, dyspnea, chest pain, or vaginal bleeding, and she had no urinary symptoms. Her past medical history was significant for an evacuation of a complete hydatidiform molar pregnancy 1 month prior to presentation. The pathology report from her surgery showed gestational trophoblastic disease consistent with a 46-XX hydatidiform mole. Her initial pre-operative human chorionic gonadotropin (HCG) level had been >225,000 mIU/mL and had fallen post-operatively but had not yet reached zero by her report. She denied tobacco, alcohol, or drug use, and she had no medications or allergies.
Physical examination revealed an ill-appearing young woman who was anxious and uncomfortable. Her vital signs were a pulse of 126 beats per minute, temperature of 36.5 °C (97.7 °F), respiratory rate of 18 breaths per minute, blood pressure of 116/82 mmHg, and an oxygen saturation of 98% on room air. The remainder of her physical examination was unremarkable with the exception of the abdominal and pelvic examinations. Her abdomen was non-distended with bilateral inferior quadrant tenderness to palpation and mild guarding without rebound. Her pelvic examination showed scant brown fluid in the vaginal vault with diffuse and significant uterine and adnexal tenderness to palpation.
Laboratory testing demonstrated a quantitative serum HCG level of 14,552 mIU/mL. Her complete blood count was normal with the exception of a hemoglobin of 10.9 g/ dL (reference range 11.7–16.1 g/dL). A bedside focused abdominal ultrasound was performed and demonstrated peri-cystic free fluid without evidence of free fluid elsewhere in the abdomen. Subsequently, a computed tomography (CT) with intravenous (IV) contrast of her chest, abdomen, and pelvis showed pelvic fluid consistent with blood surrounding a thickened and heterogeneous uterus. There was active peri-uterine contrast extravasation concerning for neoplastic process eroding into the uterine vasculature (Figure 1), and 20 discrete pulmonary nodules were seen (Figure 2).
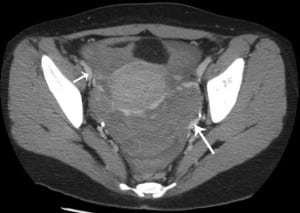
Computed tomography of the abdomen and pelvis with intravenous contrast showing free fluid in the pelvis and active peri-uterine contrast extravasation (arrows) concerning for neoplastic process eroding into the uterine vasculature.
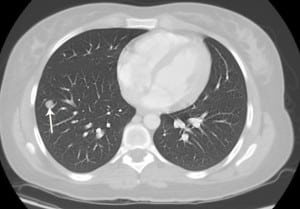
Chest computed tomography demonstrating multiple discrete pulmonary nodules (arrow).
Resuscitation with 2 liters of IV normal saline and supportive care were provided in the ED. The patient’s pulse remained tachycardic between 100–120 beats per minute, but she never became hypotensive in the ED. Large bore access was obtained bilaterally and a type and cross was sent in the ED, but the transfusion was not given until she was in the operating room. Gynecology consultation was emergently obtained, and the patient was taken directly to the operating room where she required a hysterectomy and 4 units of packed red blood cells. Her pathologic specimen demonstrated an invasive complete hydatidiform mole with foci of trophoblastic proliferation within the uterus and in the pelvis concerning for choriocarcinoma. She was started on a chemotherapy regimen post-operatively for her metastases. She did not require further surgical intervention and her choriocarcinoma went into complete remission as of follow up 6 months after chemotherapy.
DISCUSSION
Gestational trophoblastic neoplasia (GTN) comprises a group of aggressive fertilization disorders characterized by invasion of the uterine endometrial and myometrial layers by malignant trophoblastic cells. GTN includes 4 distinct pathologic diseases: choriocarcinoma, persistent/invasive hydatidiform mole, placental site trophoblastic tumor, and epithelioid trophoblastic tumor.2,3 GTN most commonly develops following a complete hydatidiform molar pregnancy, but can potentially occur after any form of pregnancy (live birth, miscarriage, or termination) and very rarely without a documented preceding gestation.2,4
The majority of cases of malignant GTN occur following a complete molar pregnancy. Persistent/malignant GTN following a complete hydatidiform mole occurs in 10–28% of cases even after surgical evacuation.1 The risk for malignant sequelae after a partial molar pregnancy is significantly less at 3–5%.5 Invasive GTN invades the myometrium or adjacent structures and can penetrate the uterine mantle causing uterine rupture and hemoperitoneum, as it did in our patient. Metastatic GTN, however, is rare after the complete evacuation of a molar pregnancy (4%), and while it only occurs in approximately 1 in 30,000 non-molar pregnancies, it is overall seen more frequently after a non-molar pregnancy.6,7
Risk factors for post-molar GTN include an HCG level greater than 100,000 mIU/mL, large theca lutein cysts (>6cm), age over 40, a history of previous GTD, and excessive uterine enlargement for presumed dates.8–10 Many patients with these risk factors for GTN are recommended for post-evacuation chemoprophylaxis, although it is not recommended for all patients unless they are known to be at high risk.
In order to monitor persistent GTN following evacuation in low risk patients, levels should be followed weekly until 3 consecutive normal values are obtained. The HCG level should return to zero in non-molar pregnancies and abortions within 2–4 weeks.11,12 HCG levels return to normal for approximately half of molar pregnancy patients within 6–14 weeks.13 It is currently recommended that monthly HCG levels are checked for 6 months after the level has returned to zero because of the risk of persistent disease. GTN should be suspected and further investigated in the setting of a preceding molar pregnancy when post-evacuation HCG levels plateau or rise. The vast majority of these cases of GTN are invasive moles with choriocarcinomas comprising less than 10% of cases.14
Our patient presented with post-partum vaginal bleeding and peritonitis secondary to a ruptured uterus and blood in her pelvis. When cases like these occur, they tend to happen when a patient does not adhere to the strict follow-up regimen.15 Because most patients with a known molar pregnancy have regular HCG testing, those with malignant GTN are often diagnosed before symptoms occur or when repeat HCG levels are abnormal. However, if symptoms occur, or the patient had a non-molar gestation and was not being monitored post-partum, the most common symptom is post-partum (or post-operative) menorrhagia.2 In women of reproductive age with abnormal vaginal bleeding more than 6 weeks post-partum, persistent GTN should be considered.16 Uterine and adnexal enlargement on pelvic examination may also be present, and peritoneal signs are possible with the presence of intra-abdominal bleeding.
If there are metastases at the time of presentation, the symptoms will vary depending on the location of the metastatic disease. Metastatic GTN is almost always due to choriocarcinoma,4 as our patient had. Lung metastases are the most common site, occurring in up to 80% of metastatic choriocarcinoma cases. Other less common sites include the vagina, pelvis, liver, and brain, but concurrent lung metastases frequently are present as well.17 Metastatic GTN is often asymptomatic,17 but because this disease may metastasize quickly, some patients may present with cough, dyspnea, or hemoptysis as a result of lung metastases or from embolization of molar/trophoblastic tissue during or after evacuation which leads to extensive infiltrates and respiratory distress.18
Diagnostic imaging may begin with an initial ultrasound of the pelvis, but CT of the abdomen and pelvis is recommended to evaluate for the extent of the disease.19,20 Imaging of other symptomatic systems is appropriate, particularly if there is concern for choriocarcinoma, which frequently present with metastases. Typically, a screening chest radiograph is sufficient to determine the presence of metastases initially in the ED, but chest CT will find metastases that are not seen on plain radiograph.19 Magnetic resonance imaging is also of particular importance in assessing for cerebral metastases and for help with staging lesions in the abdomen and pelvis.20
Management of this disease in the ED is largely supportive and requires immediate gynecologic consultation so that treatment can begin immediately. Our patient is the first reported case in the emergency literature of uterine rupture as the presenting symptoms, but it is a known potential complication of invasive and highly vascular malignant GTN. If there is significant enough malignant invasion to cause severe uterine bleeding or rupture, a hysterectomy may be emergently required to control potentially life-threatening hemorrhage.21 Long-term treatment involves chemotherapy and possible surgical resection of the other metastatic lesions as indicated.
The prognosis for malignant GTN is dependent upon the type of GTN and the stage of invasion and degree of metastasis.3 Overall, the prognosis for properly treated metastatic choriocarcinoma is worse (80–90% survival) than other types of GTN (almost 100% survival).3 Early recognition and treatment in the asymptomatic post-molar patient is the key to the high rate of cure, which approaches 100% in early stages.3 Because many choriocarcinoma patients present initially with metastatic disease, the prognosis is worse.
CONCLUSION
Metastatic GTN in the form of choriocarcinoma is a rare diagnosis to make primarily in the ED because the patients at risk for this disease are often diagnosed during routine screening. Persistently elevated HCG levels are the hallmark laboratory finding of this disease. Emergency physicians should be aware of the risk of metastatic GTN following molar pregnancies and non-molar pregnancies when there is significant post-partum vaginal bleeding or unexplained uterine or adnexal enlargement. Recognition of metastatic GTN and screening for other locations of metastases should be performed in the ED as well as supportive and resuscitative care if life threatening complications occur, such as our patient’s ruptured uterus. Overall, definitive care for GTN is the purview of the gynecologist, although emergency physicians should be aware of the risks for and be able to recognize the patient with this potentially lethal disease.
Footnotes
Address for Correspondence: David I. Bruner, MD. Naval Medical Center Portsmouth, Emergency Department, 620 John Paul Jones Cricle, Portsmouth, VA 23708. Email: dibruner@yahoo.com.
Submission history: Revision received January 13, 2013; Submitted April 9, 2013; Accepted April 11, 2013
Conflicts of Interest : By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1 Berkowitz RS, Goldstein DP Chorionic tumors. N Engl J Med. 1996; 335:1740-1748
2 Thomakos N, Rodolakis A, Balitosos P Gestational trophoblastic neoplasia with retroperitoneal metastases: A fatal complication. World J Surg Oncol. 2010; 8:114-119
3 Lurain JR Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011; 204:11-18
4 Seckl MJ, Sebire NJ, Berkowitz RS Gestational trophoblastic disease. Lancet. 2010; 376:717-729
5 Hancock BW, Nazir K, Everard JE Persistent gestational trophoblastic neoplasia after partial hydatidiform mole incidence and outcome. J Reprod Med. 2006; 51:764-766
6 Soper JT, Mutch DG, Schink JC American College of Obstetricians and Gynecologists. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol Oncol. 2004; 93:575-585
7 Berkowitz RS, Goldstein DP Current management of gestational trophoblastic diseases. Gynecol Oncol. 2009; 112:654-662
8 Berkowitz RS, Goldstein DP, DuBeshter B Management of complete molar pregnancy. J Reprod Med. 1987; 32:634-639
9 Montz FJ, Schlaerth JB, Morrow CP The natural history of theca lutein cysts. Obstet Gyncol. 1988; 72:247-251
10 Berkowitz RS, Goldstein DP Pathogenesis of gestational trophoblastic neoplasms. Pathobiol Annu. 1981; 11:391-411
11 Steier JA, Bergsjo P, Myking OL Human chorionic gonadotropin in maternal plasma after induced abortion, spontaneous abortion, and removed ectopic pregnancy. Obstet Gynecol. 1984; 64:391-394
12 Korhonen J, Alfthan H, Ylostalo P Disappearance of human chorionic gonadotropin and its alpha-and beta-subunits after term pregnancy. Clin Chem. 1997; 43:2155-2163
13 van Trommel NE, Massuger LF, Schijf CP Early identification of resistance to first-line single-agent methotrexate in patients with persistent trophoblastic disease. J Clin Oncol. 2006; 24:52-58
14 Kohorn EI The new FIGO 2000 staging and risk factor scoring system for gestational trophoblastic disease: Description and critical assessment. Inter J Gynecol Cancer. 2001; 11:73-77
15 Kennedy AW Persistent nonmetastatic gestational trophoblastic disease. Semin Oncol. 1995; 22:161-165
16 Tidy JA, Rustin GJ, Newlands ES Presentation and management of choriocarcinoma after non-molar pregnancy. Br J Obstet Gynaecol. 1995; 102:715-719
17 Berkowitz R, Goldstein D . Novak’s Gynecology. 2002;
18 Kumar J, Ilancheran A, Ratnam SS Pulmonary metastases in gestational trophoblastic disease: A review of 97 cases. Br J Obstet Gynaecol. 1988; 95:70-74
19 Garner EI, Garrett A, Goldstein DP Significance of chest computed tomography findings in the evaluation and treatment of persistent gestational trophoblastic neoplasia. J Reprod Med. 2004; 49:411-419
20 Allen SD, Lim AK, Seckl MJ Radiology of gestational trophoblastic neoplasia. Clin Radiol. 2006; 61:301-313
21 Chao A, Lin CT, Chang TC Choriocarcinoma with diffuse intra-abdominal abscess and disseminated intravascular coagulation: A case report. J Reprod Med. 2002; 47:689-692


