| Author | Affiliation |
|---|---|
| Matthew Chang, MD | Maimonides Medical Center, Emergency Department, Brooklyn, New York |
| John Marshall, MD | Maimonides Medical Center, Emergency Department, Brooklyn, New York |
We describe a 65-year-old man who presented to the emergency department with acute cerebral air embolism after receiving computed tomography guided lung biopsy. The patient presented with muscle strengths of 0/5 in left arm and 3/5 in left leg, aphasia, and a right pneumothorax. Our concern was to improve the patient’s neurological function, while ensuring stability in a patient who possibly had systemic air embolism and a penumothorax, which is a contraindication to hyperbaric treatment. To that end, we treated the patient with therapeutic hypothermia for 24 hours. The patient went on to recover full neurological functions.
CASE
A 65-year-old man presented to the emergency department (ED) after a computed tomography (CT) guided lung biopsy by interventional radiology with an acute cerebral air embolism. Prior to presentation, the patient had experienced progressive dyspnea for the prior few months and had an outpatient CT that revealed a lung mass. The patient subsequently presented to the interventional radiology suite for a CT guided lung biopsy, which later revealed adenocarcinoma. He received Versed and fentanyl for the procedure but remained alert and conversive during the procedure.
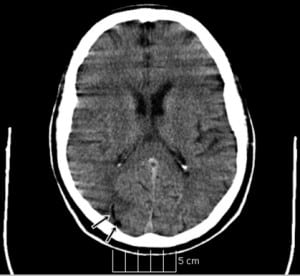
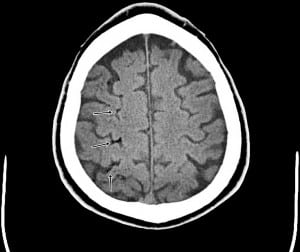
Shortly after the procedure, the patient became unresponsive. The rapid response team was activated immediately, but prior to their arrival, the patient became more alert. Upon assessment, the patient was noted to be aphasic, with left hemipareiss. A head CT was done and revealed right middle cerebral artery watershed area air embolism and hypodensity consistent with acute infarct (Figures 1 and and2).2). The chest CT showed no evidence of air embolism in the thoracic vasculature, but demonstrated a right pneumothroax, and a chest tube was inserted. The patient was not oriented to self, time, and place, but could follow simple commands intermittently. At that point, which was roughly about 30 minutes after the initial onset of symptoms, the patient was transported to the ED.
Upon initial assessment in the ED, the patient had the following vital signs: temperature of 99.4°F, pulse of 86, respiratory rate of 18, and blood pressure of 120/91. The blood glucose obtained by finger stick was 149. The patient was hemodynamically stable, alert, speaking a few words in a confused manner, and was not following simple commands. Physical exam revealed bilateral breath sounds, with slightly decreased breath sounds on the right and a right-sided pigtail catheter visibly in place. On neurological exam, the patient was noted to be flaccid in the left arm, with 0/5 in motor strength and not responding to noxious stimuli. He had 3/5 in muscle strength on the left lower extremity and was responding sluggishly to noxious stimuli.
Throughout his assessment, the patient was placed in Trendelenburg position while receiving 100% oxygen via nonrebreather mask. Hyperbaric oxygen treatment was entertained during evaluation of the patient. However, the patient presented with a concomitant pneumothorax, which is a contraindication that could be exacerbated by hyperbaric treatment. In addition, although the patient was stable upon presentation, the patient presented acutely following air embolism introduced into the vasculature and needed to be in a closely monitored setting, such as in the ED, that was prepared for resuscitation.
We, therefore, proceeded with the decision to intubate the patient and began inducing therapeutic hypothermia for neuroprotection. We used a commercial cooling device, and the target temperature was set at 31° to 33°C with the intention of maintaining the patient’s temperature at 33°C for 24 hours. The patient was also given boluses of 4 mg lorazepam and 10 mg vecuronium and started on a titratable midazolam drip to prevent shivering and maintain sedation. The patient was maintained on therapeutic hypothermia for 24 hours in the surgical intensive care unit, and the patient was extubated on hospital day 2.
Upon initial assessment following extubation, the patient was alert, oriented, and exhibited 2/5 muscle strength in the left upper extremity and 3/5 muscle strength in the left lower extremity, which was an improvement from the left upper extremity muscle strength of 0/5 on initial presentation to the ED. The patient began physical therapy daily and continued to show improvement neurologically. A repeat head CT showed resolution of air emboli on hospital day 4, with evolving right parietal-occipital hypodensity. On hospital day 5, the patient was assessed to have 4/5 in muscle strength in left upper and lower extremities, and left lower extremity muscle strength improved to 5/5 on hospital day 6. With continued effort in encouragement of out-of-bed activities and physical therapy, patient’s left upper extremity muscle strength improved to 5/5 on hospital day 8. Overall, the patient made a full recovery neurologically and was later discharged on hospital day 21. The delay in discharge was mainly due to the patient developing Clostridium difficileinfection.
DISCUSSION
Air embolism is a known, rare complication of thoracic needle aspiration and biopsy. Its incidence has been noted to be low ranging from 0.02% to 0.4%.1–3 The standard treatment for air embolism is to immediately initiate 100% oxygen in order to facilitate nitrogen diffusion into the blood serum and increase the rate of resorption of air.4 There is also evidence that suggests therapeutic advantage by placing the patient in supine position.5 Furthermore, hyperbaric oxygen therapy is given in order to increase the diffusion gradient between the air bubble and surrounding tissues.5
Therapeutic hypothermia after cardiac arrest has been extensively studied and acknowledged for its neuroprotective effect following reperfusion of cerebral tissue.6,7 Therapeutic hypothermia is thought to suppress many of the chemical reactions associated with reperfusion injury. These reactions include free radical production, excitatory amino acid release, and calcium shifts, which in turn can lead to mitochondrial damage and apoptosis.8 Although the evidence is not as conclusive as in the situation of postcardiac arrest, there have also been numerous suggestions that therapeutic hypothermia could be beneficial in the case of acute ischemic stroke, especially when the onset of the stroke is recent and reperfusion is anticipated within the timeframe that cerebral tissue is still salvageable.9–13
We’d like to point out that the main difference between using therapeutic hypothermia in patients with return of spontaneous circulation (ROSC) following cardiac arrest and in patients who suffered ischemic stroke is that patients with ROSC after cardiac arrest have a more defined pathophysiology with a progression from absent or poor perfusion during arrest to reperfusion during spontaneous circulation. After cardiac arrest, the evidence is clear that cerebral reperfusion has occurred when there is a palpable pulse and perfusing blood pressure. In contrast, it is difficult to determine the amount of perfusion and especially reperfusion following an ischemic stroke caused by an embolus or thrombus. Ultimately, reperfusion is dependent on a variety of variables, including the size and composition of the obstruction, which affect the rate of resolution.
Unlike a solid embolus, an air embolus by definition has properties of gas, such as diffusion and resorption. We believe that the intrinsic properties of gas suggest that the resolution of an air embolus and subsequent reperfusion could be expected to occur in a more timely manner when compared to the typical acute ischemic stroke, in which reperfusion is dependent on the resolution of the solid obstruction.
In addition to neuroprotective benefits during reperfusion, 1 article suggests that intra-ischemic cooling reduces infarct size under animal models.9 This article further suggests that tissue salvage is commonly achieved when hypothermia is initiated within 60 minutes of stroke onset and less effective when cooling is delayed.
There have also been other case reports of air embolism treated with therapeutic hypothermia.14–16In those cases, the patients were under cardiopulmonary bypass and had air inadvertently introduced into the vasculature. Consistently, their patients received suction to remove the air mechanically from the vasculature as well as cardiac massage to expel air in 1 case.16 The patients were then cooled using cardiopulmonary bypass respectively to 22°C for 1 hour, 24°C for 40 minutes, and 20°C for about 10 minutes.
Although all 3 cases went on to describe favorable neurological outcomes in their patients, there was no radiological evidence, unlike our current case, to indicate that their patients sustained cerebral air embolisms initially, and there was also no clinical evidence that their patients sustained acute neurological compromise since the patients were not alert and were not examined. One can only infer in their cases that therapeutic hypothermia improved neurological outcomes by assuming that there was neurological compromise in the first place. Furthermore, their patients received mechanical air removal prior to therapeutic hypothermia. Therefore, it is uncertain if the favorable neurological outcomes should be attributed to the mechanical air removal or to the therapeutic hypothermia, assuming that there was in fact air embolism remaining following mechanical air removal.
In contrast, the current case describes a symptomatic patient presenting with an acute cerebral air embolism diagnosed clinically by exam and radiologically by CT, in which therapeutic hypothermia is the only primary intervention.
Footnotes
Supervising Section Editor: Kurt R. Denninghoff, MD
Submission history: Submitted October 19, 2010; Revision received March 21, 2011; Accepted April 11, 2011
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.4.6659
Address for Correspondence: Matthew Chang, MD
Maimonides Medical Center, Emergency Department, 4802 10th Ave, Brooklyn, NY 11219
E-mail: chang_matt@yahoo.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Hiraki T, Fujiwara H, Sakurai J, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest. 2007;132:684–690. [PubMed]
2. Richardson C, Pointon K, Manhire A, et al. Percutaneous lung biopsies: a survey of UK practice based on 5444 biopsies. Br J Radiol. 2002;75:731–735. [PubMed]
3. Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–64. [PubMed]
4. Bhatia S. Systemic air embolism following CT-guided lung biopsy. J Vascular Intervent Radiol.2009;20:709–711. [PubMed]
5. Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000;342:476–482. [PubMed]
6. Bhatia S. Systemic air embolism following CT-guided lung biopsy. J Vascular Intervent Radiol.2009;20:709–711. [PubMed]
7. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. [PubMed]
8. Nolan JP, Morley PT, Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation. Circulation. 2003;108:118. [PubMed]
9. Van der Worp HB, Sena ES, Donnan GA, et al. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. [PubMed]
10. Linares G, Mayer SA. Hypothermia for the treatment of ischemic and hemorrhagic stroke. Crit Care Med. 2009;37:S243–S249. [PubMed]
11. Hemmen TM, Lyden PD. Multimodal neuroprotective therapy with induced hypothermia after ischemic stroke. Stroke. 2009;40:126–128. [PMC free article] [PubMed]
12. Dawson J, Walters M. New and emerging treatments for stroke. Brit Med Bull. 2006;77–78:87–102. [PubMed]
13. Krieger D, Yenari M. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004;35:1482. [PubMed]
14. Diethrich EB, Koopot R, Maze A, et al. Successful reversal of brain damage from iatrogenic air embolism. Surg Gynecol Obstet. 1982;154:572–575. [PubMed]
15. Spampinato N, Stassano P, Gagliardi C, et al. Massive air embolism during cardiopulmonary bypass: successful treatment with immediate hypothermia and circulatory support. Ann Thorac Surg. 1981;32:602–603. [PubMed]
16. Gomes WJ, Strisiver DA, Penco AJ, et al. Successful treatment of accidental air embolism in warm heart surgery. Asian Cardiovasc Thorac Ann. 2003;11:68–69. [PubMed]


