| Author | Affiliation |
|---|---|
| Emily E. Merchant, MD | Madigan Army Medical Center, Department of Emergency Medicine |
| Sara W. Johnson, BS | Keck School of Medicine of the University of Southern California, Department of Emergency Medicine |
| Phu Nguyen, MD | Bayne-Jones Army Community Hospital |
| Christopher Kang, MD | Madigan Army Medical Center, Department of Emergency Medicine |
| William K. Mallon, MD | Keck School of Medicine of the University of Southern California, Department of Emergency Medicine |
ABSTRACT
Takotsubo cardiomyopathy (TCM) is an unusual form of acute cardiomyopathy showing left ventricular apical ballooning. It is often triggered by intense physical or emotional distress. We report here four cases of TCM and a review of the literature on the topic.
INTRODUCTION
First described in Japan in 1990 by Sato et al,1 Takotsubo Cardiomyopathy (TCM) is an acute cardiac condition that involves left ventricular apical ballooning and mimics acute myocardial infarction (MI). It is also known as ‘transient left ventricular (LV) apical ballooning syndrome,’ ‘takotsubo-like left ventricular dysfunction,’ ‘ampulla cardiomyopathy,’ ‘stress-induced cardiomyopathy,’ and ‘broken heart syndrome.’ In Japanese, “tako tsubo” translates to “octopus pot,” which is a fishing jar with a narrow neck and wide base used to trap octopus, and describes the visual appearance of the heart on left ventriculography.
TCM patients present with symptoms of chest pain, electrocardiograph (ECG) ST-segment elevation, and cardiac markers consistent with an acute coronary syndrome (ACS). However, angiography finds no significant coronary stenosis, and the LV apex is found to balloon, which usually resolves in weeks. The syndrome appears to be triggered by emotional or physical stress. TCM is being diagnosed more frequently, possibly because of increasingly stressful times and public attention to ACS. Although well known in cardiology, awareness of this entity is still developing in emergency medicine (EM). It is important to consider this diagnosis, as patients may present to the emergency department (ED) with what appears to be uncomplicated ACS. We discuss four patients with chest pain, ECG changes and cardiac markers consistent with ACS, who were diagnosed with TCM.
CASE 1
A 67-year-old Caucasian female was sent from a clinic by ambulance to the ED for chest pain longer than 24 hours and ST elevations on ECG. Her chest pain began the previous day while mowing her lawn. It was 5/10, “stinging” in character, located substernally, and associated with shortness of breath (SOB), and radiated to her left arm. After rest, her SOB and chest pain resolved over one hour, but she continued to have intermittent chest discomfort throughout the day and again the next morning. At the primary care physician office, ECG was “concerning.”
In the ED, vital signs were: blood pressure (BP) of 140/86 mm Hg, pulse of 86 beats/min, respirations of 14 breaths/min, oxygen saturation (O2 sat) of 100% on 2 liters/minute via nasal cannula, and temperature of 37.1°C. She denied active medical issues but reported she had a rib resection in 1970 for thoracic outlet syndrome. Cardiac risks included age and 20 pack-year smoking history. When asked about family history for heart disease, she tearfully stated that the day before onset of symptoms, her sister had died of a heart attack at age 64. Apart from anxiety, her physical exam was otherwise unremarkable.
Initial ECG showed normal sinus rhythm (NSR), rate of 79 beats/min; small Q waves in inferior leads with T-wave inversions in leads II, III, and AVF; a trend toward ST elevation in lead V3; poor R wave progression; Q waves in V4 and V5, with a very small Q wave in V6; and deep T-wave inversions in V3, V4, and V5, with a small degree of T wave inversion in V6. A chest x-ray revealed no acute disease. Lab results were troponin I of 2.5 ng/ml (normal, 0.0–0.04 ng/ml), creatine kinase (CK) of 24 ng/ml (normal for females, 30–135 U/L; for males, 55–170 U/L), CK-MB quotient of 11 (normal 0.3–4.0 ng/ml), and a white blood count 11,200/mm3. Serum electrolytes and coagulation studies were normal.
The patient had immediate cardiac catheterization that revealed no source of cardiac ischemia. Left ventriculography revealed significant apical segment akinesis. Follow-up echocardiogram seven days later showed normal LV size and function with ejection fraction (EF) of 78%. Apical wall motion was normal, but the base still appeared to contract more vigorously than the apex.
CASE 2
An 86-year-old Asian female presented to the ED with chest pain of ten hours duration, which began while watching television. The pain was 9/10, substernal and non-radiating, pressure-like, without associated symptoms. Vital signs were: BP of 185/88 mm Hg, pulse of 71 beats/min, respirations of 20 breaths/min, O2 sat of 98% on room air, and temperature of 35.7°C. She reported medical history of hypertension, hypothyroidism, gout, and a hysterectomy. Cardiac risks included age, hypertension, and family history of coronary disease. She never smoked and reported a healthy diet and exercise five times per week. Her daughter stated that her mother had been under extreme stress due to sudden accidental death of her son two weeks ago. Her physical exam was unremarkable.
Initial ECG revealed a NSR with ST elevation in V2 and V3, ST depression in V4 and V5, and T wave inversions in inferior and precordial leads. Chest radiograph revealed no acute disease. Lab studies showed troponin I of 3.23 ng/ml and a white blood cell count 12,000/mm3. Serum electrolytes were normal.
She had cardiac catheterization with left ventriculography, which showed mid-anterior and apical akinesia with preserved anterobasal and posterobasal function, with an EF of 30%. Coronary arteries were unremarkable Echocardiogram showed apical akinesis with reduced LV function with EF of 34%. The mid septum showed marked hypertrophy with a thinned apex.
CASE 3
A 76-year-old Caucasian female presented to the ED with chest pain of 30 minutes duration. This began 20 minutes after she received the news that her husband was in critical condition in the intensive care unit (ICU) and needed emergent surgery. The pain was described as 5/10 nonradiating, substernal, with associated lightheadedness.
Vital signs were: BP of 110/65 mm Hg, pulse of 72 beats/min, respirations of 24 breaths/min, O2 sat of 96% on room air, and temperature of 36°C. Past medical history included osteoporosis and mitral valve prolapse. Cardiac risks included age and 45 pack-years smoking, but she quit 28 years ago. Her only medication was alendronate. Physical exam revealed a 2/6 systolic cardiac at the apex.
Initial ECG showed NSR with ST elevation in leads II, III, AVF, V2–V6, poor R wave progression, and Q waves in V2 and V3. A right-sided ECG showed no ST segment elevation in V4R. Chest radiograph showed mild pulmonary edema. Complete blood count and electrolytes were normal, but troponin I was elevated at 0.15 ng/ml.
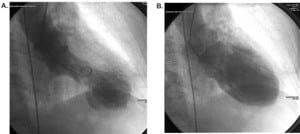
The patient immediately had cardiac catheterization with left ventriculography. This revealed EF of 20% with only basal kinesis. Coronary arteries were unremarkable. Cardiac catheterization (Figure 1) showed severe anterior-apical akinesis with compensatory inferior-posterior hyperkinesis.
CASE 4
A 42-year-old Caucasian female presented with a two-hour history of chest pain. She had just been told that one of her sons had died in a car accident and had visited her other son who was in the (ICU) in critical condition. Her chest pain began during the ICU visit and she was brought to the ED. She had a history of seizures, anxiety disorder, fibromyalgia, irritable bowel syndrome, and gastroesophageal reflux disease. The patient had one other episode of chest pain three months prior, but MI was ruled out.
In the ED, vital signs were: BP of 101/78 mm Hg, pulse of 109 beats/min, respirations of 20 breaths/min, O2 sat of 97% on room air, with pain 10/10. She was awake and alert, diaphoretic and vomited three times. The physical exam was normal. An ECG showed ST elevation in leads I, aVL, and V1–V3, with a rate of 98 beats per minute and a QTc interval of 437 ms (Figure 2a).
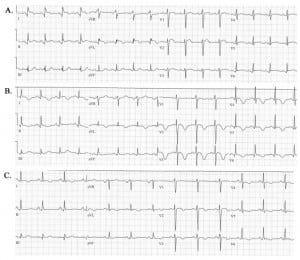
Electrocardiograms of patient showing (A) ST-segment elevation is leads V1–V3, I and aVL at 15 minutes after presentation, (B) T-wave inversion in leads V2–V6, I and aVL and a lengthened QT at one day after presentation, and (C) improvement of the T-wave inversion and normalization of ST-elevation at four days after presentation.
Initial cardiac markers were elevated with a troponin I of 5.1 ng/ml (normal, 0.0–0.3 ng/ml), a total CK of 173 U/L (normal, 25–145 U/L), and a CK-MB level of 24.7 ng/ml (normal, 0.0–5.0 ng/ml).
Emergent catheterization revealed normal coronary arteries, with a right-dominant system, and severe hypokinesis of the LV anteroapical wall, and mid to distal septum with apical ballooning. Echocardiograms performed at day 1 (Figure 3) and 4 showed a severely hypokinetic LV anteroapical wall and mid-to distal septum.
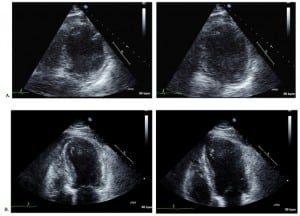
Transthoracsic echocardiogram showing (A), on day one, a severely hypokinetic left ventricular anteroapical wall and mid to distal septum during systole (left) and diastole (right) and (B) on day four, some minor improvements to the wall motion abnormalities during systole (left) and diastole (right).
DISCUSSION
Etiology
Although the clinical presentation of TCM may be identical to ACS, the suspected pathogeneses of TCM and ACS differ greatly. The etiology of Takotsubo cardiomyopathy remains speculative. Proposed mechanisms include: (1) multivessel coronary artery spasm, (2) impaired cardiac microvascular function, and (3) endogenous catecholamine induced myocardial stunning and microinfarction.
Lacy et al2 have shown that during a mental stressor of simulated public speaking patients have a decrease in coronary artery diameter, which returned to baseline after five minutes. Furthermore, these changes were similar in patients with and without coronary artery disease. Physicians who originally reported on and named ‘Takotsubo cardiomyopathy’ have also shown that approximately 70% of TCM patients had coronary spasm during a provocation test.3 They suggested that either simultaneous multivessel coronary artery spasm or microvascular spasm may contribute to the onset.
Sadamatsu et al4 reported two cases of patients with apical wall motion abnormalities with reduced coronary flow reserves not due to coronary stenosis. They postulated that this reduction in reserve may be due to microvascular dysfunction. Kurisu et al5 and Bybee et al6 showed that patients with transient LV apical ballooning syndrome have higher thrombolysis in myocardial infarction (TIMI) frame counts during the acute period than control patients. An elevated TIMI count has been associated with increased coronary microvascular resistance.7 Bybee et al6 suggested that the dysfunction of coronary microvasculature may play a role in the pathogenesis of transient LV apical ballooning syndrome. Single photon emission computed tomography (SPECT) studies of TCM patients also show impaired myocardial microcirculation.8 Other studies have also shown that mental stress may cause endothelial dysfunction, further supporting the idea that coronary microcirculation may play a role in the etiology.9,10 However, at this time, it is unclear whether the microvascular dysfunction is a cause or result of the cardiomyopathy.
The most commonly discussed mechanism for this condition is stress-induced catecholamine release. They may be a direct toxic effect on the myocardium by changing autonomic tone, enhancing lipid mobility, calcium overload, free radical production, or increased sarcolemmal permeability.11 Akashi et al12 suggested that neurogenetically mediated myocardial stunning due to autonomic imbalance may be the cause of the LV motion abnormalities. They have shown that patients with TCM had heart rate variability (HRV) parameters consistent with impaired cardiac sympathetic nervous function at time of diagnosis. These patients showed improvement in the HRV parameters at three months follow up. Wittstein et al13 showed that stress-induced cardiomyopathy patients have statistically higher levels of catecholamines (epinephrine, norepinephrine, and dopamine) than patients with myocardial infarctions. These elevated levels may cause myocardial stunning in TCM patients. The catecholamine levels however, were not known prior to the episode of TCM or MI.
Ellison et al14 showed that high doses of isoproterenol, a β1/β2 adrenergic stimulator, cause diffuse myocyte death but spare cardiac stem cells in rats. They postulated that the sparing of stem cells may contribute to the ability of myocardium to rapidly recover from acute hyperadrenergic damage, which may be the case in TCM. Bybee et al15 reported a decreased myocardial glucose uptake in TCM patients and hypothesize this may be due to myocardial insulin resistance due to catecholamine excess. Akashi et al16,17 reported an increased myocardial 123I-metaiodobenzlguanidine (123I-MBG) washout rate (WR) in TCM patients. An increased WR indicates increased norepinephrine release from sympathetic nerve endings or increased clearance of 123I-MBG by extraneural tissues. The increased WR also correlated to increased plasma norepinephrine levels in the patients, further supporting the idea of catecholamine-induced myocardial stunning.
Presentation and Clinical Course
Although TCM was first named in the early 1990s in Japan, cases in Europe were not recognized until the late 1990s and not in the United States until 2003.1,18–20 Patients with TCM often present to the ED complaining of chest pain and a standard approach is prudent. It is important to remember that TCM patients may not have cardiac risk factors, except age. These patients may have had a recent normal stress test or normal cardiac catheterization, as they do not have obstructive coronary lesions. However, more serious presentations of TCM such as ventricular fibrillation or cardiogenic shock have also been reported.21,22
The epidemiology of TCM reveals several trends. Patients are typically postmenopausal Asian or Caucasian women, but investigators do not report a racial predilection. In 286 reported cases of TCM, Gianni et al21 reported 88.8% of patients were female. In 185 cases, Donohue and Movahed22 reported age 67.7 years. Of the cases that reported race, 57.2% of patients were Asian, 40% Caucasian, and 2.8% other races.
The prevalence of the syndrome is unknown, although various researchers have reported that 1.7–2.2% of patients with suspected ACS at their institutions are diagnosed with TCM.6,8,17,23 An emotional or physical stressor frequently precedes the development of symptoms (26.8% and 37.8%, respectively).21 These stressors have included: learning of the death of a loved one, bad financial news, significant arguments, legal problems, car accidents, natural disasters, exacerbation of chronic medical illness, new significant medical diagnosis, surgery and other medical procedures, staying in an intensive care unit, and use of or withdrawal from illicit or narcotic drugs.21,22,24–28 In some cases no stressor is identifiable. 22 However, patients with traditional ACS could also have proximate stressful events, but these have not been similarly quantified.
All the patients in our series had loss of a family member as a major stressor. Family and social history have not yet shown to be relevant to TCM diagnosis. Physical exam may only be significant for emotional upset. Initial ED testing should include ECG, cardiac markers basic labs, and chest radiography.
The ECG often reveals ST elevation (often precordial) during the acute phase, followed by T wave inversion, QT prolongation, and sometimes Q waves during the subacute phase.21,29,30 A few studies have shown minor ECG differences between patients with TCM and ACS.29,31 Cardiac markers are usually elevated. However, the levels tend to be lower and normalize sooner than with ACS patients.21,22 Angiography is required for diagnosis, as there is no accurate way to reliably distinguish TCM from ACS using ECG or cardiac markers.
Patients should be treated as having ACS until proven otherwise. Aspirin and heparin should be initiated, and a cardiologist consulted. The diagnosis of TCM will be made if PCI is the initial treatment. Although thrombolysis will not benefit these patients, it should not be withheld if PCI is not available and the patient otherwise meets criteria.
Coronary angiography, echocardiography, or ventriculography of TCM patients during the acute phase reveal left mid-ventricular dysfunction and apical dyskinesis or akinesis with apical ballooning. Mean EF for these patients range from 20–49%.21 LV basal hyperkinesis also occurs, and one systematic review reported 16% with transient LV outflow tract obstruction (LVOT) due to this hyperkinesis.21 Coronary arteries are either normal or show mild stenosis (<50% luminal stenosis).21,30 On follow-up studies, patients show rapid improvement in wall motion and restoration of LV function with improved EF of 60–76%.21
Acute complications occur in approximately 20% of TCM cases and include cardiogenic shock, left-sided heart failure with or without pulmonary edema, tachy- and bradyarrhythmias, LV thrombus formation or free wall rupture, and death.21–23,30,32–36 Cardiogenic shock results from pump failure or LVOT obstruction, which can cause severe mitral regurgitation. For hypotensive patients, urgent echocardiography should be performed.30 If hypotension is due to pump failure, inotropic agents should be initiated. If necessary, intra-aortic balloon counterpulsation has been utilized in TCM patients.33 It is important to recognize and diagnose TCM-related cardiogenic shock, and treat with the balloon pump because TCM patients fare much better than other causes of cardiogenic shock. If the hypotension is due to LVOT obstruction, inotropic agents will worsen the obstruction. Instead, beta-blockers and fluid resuscitation are the initial treatment.
The long-term therapy of TCM has been that of cardiomyopathy with LV systolic dysfunction: beta-blockers, ACE-inhibitors, and diuretics. Aspirin and calcium channel blockers have also been recommended.30 However, Fazio et al37 found that treatment with any of these medications does not make a significant difference in outcome. Prognosis for patients with TCM is good for those who survive the acute episode. Mortality rates have been reported by Gianni et al21 and Donohue and Movahed22 at 1% and 3.2%, respectively. Recovery time for TCM patients is generally rapid. Marked improvement in ECG findings, cardiac markers, and EF can be seen within days.13,17,26,38 Complete recovery of LV usually occurs within 1–4 weeks, although some have taken up to a year.32 Recurrence appears to be rare at 2–3%. However, as this is a newly described entity, accurate long-term data is not yet available.21,33
Diagnostic criteria for TCM have not been established. However both American and Japanese researchers have proposed similar criteria for the diagnosis of TCM. These authorities stress the important findings of: (1) Transient akinesis or dyskinesis of the apical and midventricular segments in association with regional wall motion abnormalities that extend beyond the distribution of a single epicardial vessel; (2) immediate ST segment elevation on ECG followed by T waves that become progressively more negative and a prolongation of the QT interval; (3) only a modest elevation of cardiac markers; and (4) absence on angiography of obstructive coronary artery disease or evidence of acute plaque rupture. The groups also put forth similar exclusion criteria: (1) recent significant head trauma or intracranial bleeding, (2) cerebrovascular disease, (3) pheochromocytoma, and (4) myocarditis.30,39 These criteria have not been validated, but can be used as guidelines that, when present, should raise suspicion of TCM.
Several ED circumstances may lead to TCM presentation. The ED deals with the consequences of natural disasters, and emergency physicians (EP) should be aware that this syndrome might present during or soon afterward. The syndrome may also occur in younger patients without cardiac risk factors. Some anxious or emotionally distraught patients with chest pain may be more complicated than simple anxiety disorder, and may develop dysrhythmias or shock.
The emotional distress and chest pain of family members after death notification are sometimes dismissed or managed with benzodiazepines and social work consult. While much has been written on the emotional support needed for survivors after death notification in the ED, we found no study that looked at the medical management of physical symptoms of these survivors.40–44 EPs should consider TCM in the differential diagnosis of chest pain following death notification. Increased awareness of this entity will contribute to timely diagnoses and appropriate treatment.
History
Although TCM was named in the early 1990s the idea of a stress-induced physical disorder or death has been in the literature for decades. In 1942 Walter B. Cannon45wrote a remarkable article detailing numerous accounts of so-called “Voodoo death” reported by educated and independent observers. The cases of Voodoo death occurred among many different aboriginal tribes throughout the world. The cases involved people who were “hexed” or condemned to death by medicine men or Voodoo priests. In all cases the condemned person and all his/her family and associated believed there was no escaping the death that was sure to ensue. In most of these cases poison was ruled out or unlikely as the cause of death, and Cannon postulated that intense fear could so over-activate the sympathetic and sympatho-adrenal systems that death ensued.45
In 1971 Engel46 reported on 170 cases of sudden death associated with psychological stress. He identified eight categories in which these deaths could be classified: learning of the death of a loved one, during the acute grief period, threat of loss of a loved one, during mourning or an anniversary, on loss of status or self-esteem, threat of or actual physical danger, and reunion, triumph, or happy ending. All these instances represent a situation in which the person is overwhelmingly distraught or excited and are very similar to the precipitating events reported in TCM. Engel postulated that these deaths may involve activation of both a strong sympathetic and parasympathetic response and lead to lethal cardiac events.46
Cebelin and Hirsch47 reported in 1980 the post-mortem analysis of myocardium from 15 victims who died from physical assault but whose autopsies revealed no internal injuries sufficient to cause death. Many of these patient’s (73%) hearts showed myofibrillar degeneration and “contraction band” necrosis. Cebelin and Hirsch47 postulated that the cause of death could have been a catecholamine mediated “stress cardiomyopathy.” Interestingly, Wittstein et al.13 have reported very similar endomyocardial pathologies in TCM patients. Although the mortality rate in diagnosed TCM is low, it is possible that some of these sudden unexplainable deaths during intense emotional distress represent a more-fatal version of TCM.
CONCLUSION
As a newly recognized disorder, much remains unknown about TCM, especially etiology. Other aspects are also puzzling, such as why postmenopausal women are mostly affected and why the apex of the LV is so impaired while the remainder is relatively spared. TCM is a rare but potentially fatal condition, initially indistinguishable from ACS. The EP should consider the diagnosis in patients with chest pain and a recent stressful event, especially elderly females.
Footnotes
Supervising Section Editor: Sean O. Henderson, MD
Submission history: Submitted December 13, 2007; Revision Received December 17, 2007; Accepted January 4, 2008.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Emily Merchant, MD. Madigan Army Medical Center, Bldg 9040 Fitzsimmons Drive, Department of Emergency Medicine, Tacoma, WA 98431
Email: emily_merchant@hotmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Sato H, Tateishi H, Uchida T, et al. Takotsubo type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M, editors. Clinical aspect of myocardial injury: from ischemia to heart failure. Kagaku Hyoronsha; Tokyo: 1990. pp. 56–64. [in Japanese]
2. Lacy CR, Contrada RJ, Robbins ML, et al. Coronary Vasoconstriction induced by mental stress (simulated public speaking) Am J Cardio. 1995;75:503–505.
3. Kurisu S, Sato H, Kawagoe T, et al. Takotsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction.Am Heart J. 2002;143:448–455. [PubMed]
4. Sadamatsu K, Tashiro H, Maehira N, et al. Coronary microvascular abnormality in the reversible systolic dysfunction observed after noncardiac disease. Jpn Circ J.2000;64:789–792. [PubMed]
5. Kurisu S, Inoue I, Kawagoe T, et al. Myocardial perfusion and fatty acid metabolism in patients with Takotsubo-like left ventricular dysfunction. J Am Coll Cardiol.2003;41:743–8. [PubMed]
6. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–346. [PubMed]
7. Barcin C, Denktas AE, Garratt KN, et al. Relation of thrombolysis in myocardial infarction (TIMI) frame count to coronary flow parameters. Am J Cardio. 2003;91:466–469.
8. Ito K, Sugihara H, Katoh S, et al. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mtc-tetrofosmin myocardial SPECT—comparison with acute coronary syndrome.Ann Nucl Med. 2003;17:115–122. [PubMed]
9. Spieker LE, Hürlimann D, Ruschtzka F, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105:2817–2820.[PubMed]
10. Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. [PubMed]
11. Zipes DP, Libby P, Bonow R, Braunwald E, editors. Braunwald‘s Heart Disease: A Textbook of Cardiovascular Medicine. 7. Philadelphia, PA: Saunders; 2005.
12. Akashi YJ, Barbaro G, Sakurai T, et al. Cardiac autonomic imbalance in patients with reversible ventricular dysfunction Takotsubo cardiomyopathy. Q J Med. 2007;100:335–343.
13. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. [PubMed]
14. Ellison GM, Torella D, Karakikes I, et al. Acute β-adrenergic overload produces myocyte damage through calcium leakage from ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem. 2007;282:11397–11409. [PMC free article] [PubMed]
15. Bybee KA, Murphy J, Prasad A, et al. Acute impairment of regional myocardial glucose uptake in the apical ballooning (Takotsubo) syndrome. J Nucl Cardiol.2006;13:244–50. [PubMed]
16. Akashi YJ, Nakazawa K, Sakakibara M, et al. Reversible left ventricular dysfunction “Takotsubo” cardiomyopathy related to catecholamine cardiotoxicity. J Electrocardiol.2002;35:351–356. [PubMed]
17. Akashi YJ, Nakazawa K, Sakakibara M, et al. 123I-MIBG myocardial scintigraphy in patients with “Takotsubo” cardiomyopathy. J Nucl Med. 2004;45:1121–1127. [PubMed]
18. Dote K, Sato H, Uchida T, et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol. 1991;21:203–214. [PubMed]
19. Pavin D, Le Breton H, Daubert C. Human stress cardiomyopathy mimicking acute myocardial syndrome. Heart. 1997;78:509–511. [PMC free article] [PubMed]
20. Seth PS, Aurigemma GP, Krasnow JM, et al. A syndrome of transient left ventricular apical wall motion abnormality in the absence of coronary disease: a perspective from the United States. Cardiology. 2003;100:61–66. [PubMed]
21. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or Takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. [PubMed]
22. Donohue D, Movahed MR. Clinical characteristics, demographics, and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;10:311–316.[PubMed]
23. Matsuoka K, Okubo S, Fujii E, et al. Evaluation of the arrhythmogenecity of stress–induced “Takotsubo cardiomyopathy” from the time course of the 12-lead surface electrocardiogram. Am J Cardiol. 2003;92:230–233. [PubMed]
24. Bruder O, Hunold P, Jochims M, et al. Reversible late gadolinium enhancement in a case of Takotsubo cardiomyopathy following high-dose dobutamine stress MRI. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.01.081. [Cross Ref]
25. Sato M, Fujita S, Saito A, et al. Increased incidence of transient left ventricular apical ballooning (so called ‘Takotsubo’ cardiomyopathy) after the mid-Niigata prefecture earthquake. Circ J. 2006;70:947–953. [PubMed]
26. Park JH, Kang SJ, Song JK, et al. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128:296–302.[PubMed]
27. Rivera JM, Locketz AJ, Fritz KD, et al. Broken heart syndrome” after separation (from oxycontin) Mayo Clin Proc. 2006;81:825–828. [PubMed]
28. Arora A, Alfayoumi F, Srinivasan V. Transient left ventricular apical ballooning after cocaine use: is catecholamine cardiotoxicity the pathologic link? Mayo Clin Proc.2006;81:829–832. [PubMed]
29. Inoue M, Shimizu M, Ino H, et al. Differentiation between patients with Takotsubo cardiomyopathy and those with anterior acute myocardial infarction. Circ J.2005;69:89–94. [PubMed]
30. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction.Ann Intern Med. 2004;141:858–865. [PubMed]
31. Kurisu S, Inoue I, Kawagoe T, et al. Time course of electrocardiographic changes in patients with Takotsubo syndrome—comparison with acute myocardial infarction with minimal enzymatic release. Circ J. 2004;68:77–81. [PubMed]
32. Giordan M, Rigatelli G, Cardaioli P, et al. Angiographic long-term follow-up of primary apical ballooning of the left ventricle. Int J Card Imag. 2006;22:349–352.
33. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol. 2001;38:11–18. [PubMed]
34. Kurisu S, Inoue I, Kawagoe T, et al. Left ventricular apical thrombus formation in a patient with suspected Takotsubo-like left ventricular dysfunction. Circ J. 2003;67:556–558. [PubMed]
35. Tibrewala AV, Moss BN, Cooper HA. A rare case of Takotsubo cardiomyopathy complicated by a left ventricular thrombus. South Med J. 2006;99:70–73. [PubMed]
36. Sasaki N, Kinugawa T, Yamawaki M, et al. Transient left ventricular apical ballooning in a patient with bicuspid aortic valve created a left ventricular thrombus leading to acute renal infarction. Circ J. 2004;68:1081–1083. [PubMed]
37. Fazio G, Pizzuto C, Barbaro G, et al. Chronic pharmacological treatment in Takotsubo cardiomyopathy. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.04.013. [Cross Ref]
38. Ibanez B, Navarro F, Cordoba M, et al. Takotsubo transient left ventricular apical ballooning: is intravascular ultrasound the key to resolve the enigma? Heart.2005;91:102–104. [PMC free article] [PubMed]
39. Kawai S, Kitabatake A, Tomoike H, et al. Guidelines for diagnosis of Takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71:990–992. [PubMed]
40. Ahrens W, Hart R, Maruyama N. Pediatric death: managing the aftermath in the emergency department. J Emerg Med. 1997;15:601–603. [PubMed]
41. Greenberg LW, Ochsenschlager D, Cohen GJ, et al. Counseling parents of a child dead on arrival: a survey of emergency departments. Am J Emerg Med. 1993;11:225–229.[PubMed]
42. Iserson KV. Notifying survivors about sudden, unexpected deaths. West J Med.2000;173:261–265. [PMC free article] [PubMed]
43. Jurkovich GJ, Pierce B, Pananen L, et al. Giving bad news: the family perspective. J Trauma. 2000;48:865–873. [PubMed]
44. Schmidt TA, Norton RL, Tolle SW. Sudden death in the ED: educating residents to compassionately inform families. J Emerg Med. 1992;10:643–647. [PubMed]
45. Cannon WB. “Vodoo” death. American Anthropologist. 1942;44:169–181.
46. Engel GL. Sudden and rapid death during psychological stress: folklore or folk wisdom? Ann Intern Med. 1971;74:771–782. [PubMed]
47. Cebelin MS, Hirsch CS. Human stress cardiomyopathy: myocardial lesions in victims of homicidal assaults without internal injuries. Hum Pathol. 1980;11:123–132.[PubMed]