| Author | Affiliation |
|---|---|
| Ray A. Grijalva, Jr., MD | University of California, Irvine School of Medicine |
| Mike Ritter, MD | Mission Hospital, Department of Emergency Medicine |
| Mark I. Langdorf, MD, MHPE | University of California, Irvine Medical Center, Department of Emergency Medicine |
ABSTRACT
This case report describes a sternoclavicular infection in an IV drug user. The history and physical exam suggested an abscess. In the emergency department (ED) the patient refused incision and drainage but did consent to simple needle aspiration. Subsequent culture of the aspirate revealed Pseudomonas aeruginosa. He was admitted for IV antibiotics. After admission, a bone scan suggested the presence of osteomyelitis. The patient refused operative débridement, but ultimately did consent to bedside incision and drainage. By day five, the fever had resolved and the patient signed out AMA. He was given a prescription for Ciprofloxacin. The patient had an unscheduled follow up in the ED five months later for an unrelated heroin overdose. Physical examination demonstrated complete resolution of the infection.
INTRODUCTION
Bacterial septic arthritis is dangerous and destructive. Early intervention is key to prevent irreversible joint damage and hematogenous spread. In the United States, the most common etiology of septic arthritis is Neisseria gonorrhea.1 Sternoclavicular joint and sacral iliac joint infections are associated with intravenous drug use (IVDU). Methicillin-resistant Staphylococcus aureus (MRSA) has surpassed Pseudomonas aeruginosa as the most common cause of sternoclavicular septic arthritis.2 While one case of sternoclavicular septic arthritis due to Staphylococcus aureus has been published in the emergency medicine literature,3 we present the first case of septic arthritis of the sternoclavicular joint due to Pseudomonas aeruginosa.
CASE REPORT
Chief Complaint
“I’m growing a breast.”
HISTORY
A 28-year-old man presented to the emergency department (ED) with a painful fluctuant swelling in the left anterior chest. He first noticed the swelling one week prior when a friend joked he was growing a breast. Since that time, the area has become progressively more swollen. He also reported fevers and chills for the past two days. The pain was worse with arm movement. The patient has used IV heroin for three years, most recently just prior to the ED visit, and most commonly injected his arms. The patient reused his needles after cleaning them in vodka. He denied any IVDU in the neck or chest and had never had similar swellings in the past. He denied skin popping and pocket shooting. Past medical history was unremarkable. The patient had never been tested for hepatitis, HIV, or sexually transmitted diseases. Last tetanus toxoid was two years ago. He denied sharing needles.
PHYSICAL EXAM
The patient appeared in mild discomfort. Vital signs were pulse of 102 beats per minute, blood pressure of 140/72 mm Hg, respiratory rate at 18 breaths per minute and temperature 99.6 °F orally.
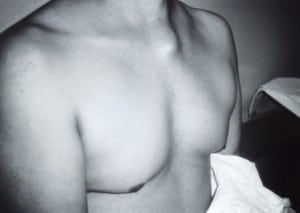
The head exam was normal; the neck revealed several small (<1cm) mobile lymph nodes primarily on the left side of the neck anterior to the sternocleidomastoid muscle. Chest exam revealed a 3 × 4 cm tender, fluctuant, nodular mass in the area of the left sternoclavicular joint (Figure). There was no overlying erythema or other skin lesions on the chest. The cardiovascular exam revealed no murmurs; peripheral pulses were strong and equal. The abdominal exam revealed normal bowel sounds; it was soft and non-tender with no organomegaly. The extremities had track marks, but no evidence of abscesses or cellulitis. Movement of the left arm caused pain localized to the swelling. There was no physical exam evidence of embolic phenomena (no Osler’s nodes, Janeway lesions, splinter hemorrhages, Roth spots, or conjunctival petechiae.)
DIAGNOSTIC TESTING
The CBC revealed a white count of 13, 500/mm3. The hemoglobin was 13.5 g/dl. Platelets were 459,000/mm3. The sedimentation rate was 97 mm/hr. Electrolytes, liver panel, renal function and urinalysis was normal. A chest radiograph was normal with no bony destruction in the area of swelling. The patient refused incision and drainage of the suspected abscess. After a lengthy discussion and education on the increasing frequency of resistant organisms found in abscesses, he consented only to a diagnostic needle aspiration. Using sterile technique, the area was prepped and draped. Two cc’s of 1% lidocaine was infiltrated subcutaneously. Then, an 18G needle was easily inserted into the center of the abscess yielding 12 cc of purulent fluid. Diagnostic studies of the aspirate included a gram stain, cell count, and culture and sensitivity. Cell count revealed 78,000 WBCs /mm3 and gram stain showed gram-negative rods. Wound culture and two blood cultures were sent.
DIAGNOSIS
Sternoclavicular septic arthritis and abscess.
TREATMENT
In the ED, the patient received IV vancomycin and ceftazidime. After admission, the orthopedic service incised, drained, and irrigated the abscess at the bedside. The initial aspirate was positive for Pseudomonas aeruginosa, sensitive to ceftazidime and ciprofloxacin. Blood cultures were negative. An echocardiogram revealed no valvular abnormalities or vegetations. HIV, hepatitis B and C testing were negative. A bone scan revealed osteomyelitis in the area of the left sternoclavicular joint and clavicle. Orthopedics further recommended surgical irrigation and débridement of the joint space, but the patient refused. The fever subsided on hospital day three and the patient signed out AMA on day five, with a prescription for 30 additional days of oral ciprofloxacin. He did not follow up at the clinic.
Although he missed his clinic follow up, he did return for further care five months later for a subsequent overdose that required treatment with naloxone. When lucid, he reported taking the entire course of Cipro because he did not want to get “shooter’s heart.” He no longer had pain or swelling in the area, or any fevers, and said he felt fine (except for the acute withdrawal symptoms).
DISCUSSION
This case illustrates the difficulty in the evaluation of some ED patients. Many IVDUs are reluctant to cooperate with a complete medical evaluation for a number of reasons: fear of narcotic withdrawal, drug intoxication, lack of health insurance, social stigma, just to name a few. Signing out against medical advice is a common problem in this population. These patients often have serious medical problems and can present a high liability to the emergency physician. Patient education is critically important. Additionally, infections in this patient population require rapid evaluation and early antibiotic administration due to the high morbidity and mortality.
Sternoclavicular abscess is a potential complication of septic arthritis of the sternoclavicular joint,4,5 found in 22% of cases. The mean age is 45 years, with 74% male.2 Patients often present with a painful swelling over the sternoclavicular joint preceded by chest pain or pain referred to the neck or shoulder. Fever and leukocytosis are of little help, as 35% are afebrile and 44% have a normal WBC.2 An erythrocyte sedimentation rate (ESR) is more sensitive at 91%,6 but merely indicates inflammation not specific to any organism.7 Bacteremia occurs in 62% of patients with sternoclavicular septic arthritis, with osteomyelitis occurring 55% of the time, and mediastinitis, 13%. Currently, Staphylococcus aureus is the most common bacteria in sternoclavicular abscess formation in IVDU patients. However, this represents a shift, as Pseudomonas aeruginosa was responsible for the bulk of cases (82%) prior to 1981, whereasStaphylococcus aureus caused most cases (77%) after 1981.8
Two factors may help explain why pseudomonas was the most common gram-negative pathogen, and why, more recently, S. Aureus has dominated. First, the IVDU drug of choice prior to 1981 was commonly pentazocine, which was frequently injected with the adjuvant, tripelennamine. This first-generation ethylenediamine antihistamine was thought to intensify the “rush” associated with pentazocine injection. One study found that tripelennamine favored the growth and survival of certain serotypes of pseudomonas.8 This, in combination with the routine use of contaminated water sources to clean needles,9,10 made pseudomonas the most common organism causing sternoclavicular septic arthritis in IVDU patients prior to 1981.2
Second, a shift in the illicit drug of choice from pentazocine to heroin more recently may have contributed to the observed shift in bacteriology. Prior to the 1980s, the commonly used pentazocine did not require heating prior to injection. Therefore, pseudomonas remained the more common offending organism. Heroin has now surpassed pentazocine use.11 Because heroin requires heating prior to injection, bacterial contaminants are eliminated. As a result, skin pathogens predominate as the most common organisms. These factors, in our opinion, help to explain both the historical predominance ofPseudomonas aeruginosa as the cause of sternoclavicular septic arthritis, as well as its replacement as the predominant organism by staphylococcus.2 Thus, this case illustrates a declining but still important diagnostic consideration.
In a recent study Staphylococcus aureus was responsible for half of all sternoclavicular joint infections, while Pseudomonas aeruginosa was responsible for only 10%.2 The reason for this predilection to the sternoclavicular joint may be the combination of surface proteins, which bind connective tissue and the proximity of the subclavian vein.11Intravenous drug use is the most common predisposing risk factor for sternoclavicular septic arthritis (21%), followed by upper extremity infection (15%), diabetes mellitus (13%), trauma (12%) and subclavian catheters (9%). However, almost one quarter of patients with sternoclavicular septic arthritis have no risk factors.2
The presentation of septic arthritis does not inform the etiology of the infection. However,Pseudomonas aeruginosa can give rise to blue-green pus. Occasionally, a breakdown product of hemoglobin called verdoglobin can be detected in pseudomonas infections by ultraviolet light in suspect wounds.12 Pseudomonas also has a characteristic “fruity-musty” odor.13 The most specific lesion for a pseudomonas infection is ecthyma gangrenosum, which is a hemorrhagic necrosis of skin that does not contain pus, and is due to pseudomonas infection.12
Treatment includes incision and drainage of a suspected sternoclavicular abscess, as well as wound and blood cultures (at least three if endocarditis is suspected). In this case, the patient would not allow incision and drainage of the abscess. Surgical incision and drainage is considered the definitive treatment of a soft-tissue abscess.14 Diagnostic needle aspiration is recommended if one is unsure of pus localization. It should not be considered a substitute for incision and drainage.15
The evaluation and management of suspected soft-tissue infections and abscesses in febrile IVDUs must be done rapidly. Diagnostic evaluation includes lab tests, blood and wound cultures, and incision and drainage of the abscess. The timely administration of empiric antibiotics should include coverage for MRSA and pseudomonas species (e.g. vancomycin and ceftazidime). Gram stain and culture of the sternoclavicular abscess will help guide antibiotic selection, especially with the increasing frequency of resistant organisms such as methicillin resistant Staphylococcus aureus (MRSA). Surgical debridement or resection should be considered if imaging studies detect osteomyelitis or spread into adjacent structures.1
Footnotes
Supervising Section Editor: Robert W. Derlet, MD
Submission history: Submitted November 2, 2007; Revision Received December 2, 2007;Accepted January 2, 2008.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for correspondence: Mark I. Langdorf, MD, MHPE, UCI Medical Center, Department of Emergency Medicine, 101 City Drive South, Route 128, Orange, CA 92868\
Email: milangdo@uci.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Kutty K, Schapira R, Van Ruiswyk J. Kochar’s Concise Textbook of Medicine. Baltimore, MD: Lippincott Williams & Wilkins; 2003. pp. 1007–1009.
2. Ross JJ, Shamsuddin H. Sternoclavicular Septic Arthritis. Medicine. 2004;83:139–148. [PubMed]
3. Hamilton J, Wylie R. Sternoclavicular Pyarthrosis. Staph aureaus only. J Emerg Med.2003;24:327–328. [PubMed]
4. Linthoud DV, Velan F, Ott H. Abscess formation in sternoclavicular septic arthritis. J Rheumatol. 1989;16:401–414.
5. Wohlgethan JR, Newberg AH, Reed JI. The risk of abscess from sternoclavicular septic arthritis. J Rheumatol. 1988;15:1302–1306. [PubMed]
6. Sapico FL, Montgomerie JZ. Vertebral osteomyelitis in intravenous drug abusers: report of three cases and review of the literature. Rev Infect Dis. 1980;2:196–206.[PubMed]
7. Calder KK, Severyn FA. Surgical emergencies in the intravenous drug user.Emergency Medicine Clinics of North America. 2003;21:1089–1116. [PubMed]
8. Botsford KB, Weinstein RA, Nathan CR, et al. Selective survival in pentazocine and triplennamine of Pseudomonas aeruginosa serotype 011 from drug addicts. J Infect Dis.1985;151:209–216. [PubMed]
9. Smith JW, Piercy EA. State of-of-the-art clinical article: Infectious arthritis. Clin Infect Dis. 1995;20:225–231. [PubMed]
10. Schoener EP, Hopper JA, Pierre JD. Injection drug use in North America. Infect Dis Clin N Am. 2002;16:535–551.
11. Lowy FD. Mecical progress: Staphlococcus aureus infections. N Eng J Med.1998;339:5202–532.
12. Brooks G, Butel J, Morse S. Medical Microbiology. Lange Medical Books/McGraw-Hill Compaines Inc.; 2004. pp. 262–264.
13. Kavic S, Cohn S. Infection based on odor. J Trauma. 1996;41(6):1077. [PubMed]
14. Meislin HW, McGehee MD, Rosen P. Management and microbiological causes of cutaneous abscesses. JACEP. 1978;7:186–191. [PubMed]
15. Roberts JR, Hedges JR. Clinical procedures in emergency medicine. 3. Philadelphia: W.B. Saunders; 1998. p. 637.