| Author | Affiliation |
|---|---|
| Christopher J. Coyne, MD | Los Angeles County + University of Southern California Health Network, Department of Emergency Medicine, Los Angeles, California |
| Ashokokkumar Jain, MD, MPH | Los Angeles County + University of Southern California Health Network, Department of Emergency Medicine, Los Angeles, California |
ABSTRACT
Pylephlebitis is a septic thrombophlebitis of the portal vein that is associated with multiple suppurative abdominal infections, such as diverticulitis, appendicitis, cholangitis, and cholecystitis. We describe a case of pylephlebitis in a patient with fever and diffuse, poorly localized abdominal pain who was eventually diagnosed with appendicitis. We aim to increase awareness of this condition among emergency physicians, as timely initiation of antibiotics and expedited surgical resection may improve outcomes in this potentially fatal disease.
CASE REPORT
The patient, a 27-year-old Hispanic male with no past medical or surgical history, presented to the emergency department (ED) with a chief complaint of fevers, chills and intermittent epigastric pain for 5 days. He stated that this pain started acutely, 30 minutes after eating dinner (steak and beans), was burning in quality, non-radiating and lasted approximately 20 minutes. This pain recurred several times over multiple days. Two days prior to arrival in the ED, the patient began experiencing subjective fevers and chills. He also reported having a single episode of vomiting, but denied diarrhea, sick contacts, recent travel, dysuria, hematuria, chest pain or shortness of breath.
On the initial physical exam the patient was found to be tachycardic (122 beats per minute), but afebrile (98.2°F) and relatively normotensive (106/59 mmHg). He was diaphoretic, but otherwise in no significant distress. His skin was mildly jaundiced and his sclera was icteric. His lungs were clear to ausculation and his cardiac exam was negative for murmurs, rubs, or gallops. His extremities were within normal limits, with no evidence of edema or rash. The patient was mildly tender in the epigastrium, but his abdomen was soft and non-distended. He had no rebound tenderness, no involuntary guarding, no Rovsing’s, Obturator or Murphy’s signs. The remainder of his abdomen was non-tender. Labs were significant for a leukocytosis of 16,000 with toxic granulations and a neutrophilic predominance. The patient was thrombocytopenic with a platelet count of 20,000/μL. His D-dimer and fibrinogen were elevated at 1428 mg/dl and 864 mg/dl, respectively. Finally, his liver function tests revealed an elevated total biliruben of 2.9 mg/dL and an elevated alkaline phosphatase of 278. The patient also received a chest radiograph and electrocardiogram, which were free of abnormalities.
The patient was treated for severe sepsis vs. septic shock. He received multiple boluses of normal saline, in addition to broad-spectrum antibiotics to cover for a suspected intra-abdominal infection. The patient responded well to these interventions, his vital signs began to normalize, and he stated that he was feeling better.
An abdominal ultrasound was ordered for further evaluation, given the patient’s epigastric pain and abnormal liver function tests. On this study, the liver appeared normal, with no intra- or extra-hepatic ductal dilatation. The kidneys, aorta, spleen and pancreas all appeared within normal limits. The gallbladder contained no stones or sludge, but the gallbladder wall was mildly thickened at 5mm. There was no evidence of peri-cholecystic fluid.
At this time, a surgical consult was placed for evaluation of possible acute cholecystitis/cholangitis. During his evaluation, the surgeon found no tenderness or distension on physical exam, but noted the abnormal lab findings, as well as the gallbladder wall thickening on ultrasound. He stated that it might have been an atypical case of acute cholecystitis, but given the constellation of abnormal lab values, he recommended a computed tomography (CT) of the abdomen/ pelvis and further medical workup.
The patient was subsequently admitted to the medical intensive care unit for continued antibiotic therapy and diagnostics. In addition to a standard infectious workup, the patient received testing for a variety of multi-systemic infections including influenza, viral hepatitis, HIV, malaria and tularemia. On hospital day 2, 2 of the 4 blood cultures grew E. coli. The patient’s antibiotics were tailored for more specific therapy and a CT of the abdomen and pelvis was obtained on hospital day 3. A preliminary report noted a dilated and thick walled retrocecal appendix with adjacent inflammation and free fluid, consistent with acute appendicitis. The radiologist noted inflammation of the gallbladder wall, but suspected that this was secondary to the adjacent inflamed appendix.
On hospital day 4, the final CT abdomen/pelvis read was submitted, which confirmed the diagnosis of appendicitis, and also noted small, branching, hypodense, tubular lucencies in the right lobe of the liver consistent with pylephlebitis (Figure). The patient was subsequently taken to the operating room for a laproscopic appendectomy. The procedure was performed without complications. Intravenous antibiotics were continued until hospital day 6 when the patient was discharged. There was no mention of pylephlebitis in the primary team notes, or in the surgical consult notes. Antibiotics were discontinued upon discharge, despite an elevated white blood cell count of 15.1 × 103/μL (16.1 ×103/μL on admission). The patient’s total bilirubin remained elevated as well (3.0 mg/dL), compared to his admission value of 2.9 mg/dL.
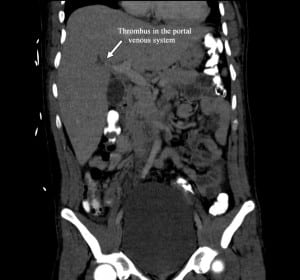
Computed tomography demonstrating a thrombus in the portal vein and therefore pylephlebitis
One week later, the patient was seen in surgery clinic. He stated that he was feeling better, with minimal pain at the surgery site, and that he was able to eat and drink without issue. His staples were removed and he was told to return as needed. He was then lost to follow up.
DISCUSSION
Pylephlebitis is defined as a septic thrombophlebitis of the portal vein and its tributaries. While diverticulitis is thought to be the leading cause of this disease, it has also been described in appendicitis, cholecystitis, hemorrhoidal disease, necrotizing pancreatitis, and various other suppurative intra-abdominal infections.1 The disease was first described by Waller in 1846 as a source of pyogenic intrahepatic abscesses.2 Prior to the advent of antibiotics, this condition was almost universally fatal. Even today, mortality from pylephlebitis can range anywhere between 30–50%. This high mortality rate is likely related to delayed diagnosis, given the low incidence of suspicion and atypical findings.3
The clinical presentation of pylephlebitis is frequently non-specific. Fever is the most common presenting symptom, followed by abdominal pain, which is often vague and poorly localized.1,2 Nausea, vomiting, scleral icterus and a tender, enlarged liver may also be present in some cases, especially in those complicated by liver abscesses.1,4 In approximately 80% of patients, blood cultures will be positive, with the most common organism being Bacteroides fragilis, followed by E. Coli and Steptococcus sp.1,2,4 Liver function tests may be abnormal in some cases, but are generally normal. Leukocytosis is typical, but may be absent in neutropenic patients with hematologic malignancies, who are potentially at an increased risk for pylephlebitis given their immuno-compromised and hypercoagulable states.5
The diagnosis of pylephlebitis is generally made through either doppler ultrasound or CT. Ultrasound with color doppler is a fairly sensitive test for thrombosis of the portal vein. Typical sonographic features include echogenic material in the lumen of the vein, as well as distension of the thrombosed segment. Ultrasound may also be used as a follow-up exam to demonstrate recanalization. As with all ultrasonagraphic studies, however, the diagnostic accuracy is generally operator dependent.5,6 CT is the most-used diagnostic modality for this disease, with typical findings being a suppurative source, in addition to a thrombus in the portal vein or its tributaries.2,5 In certain cases, serial CTs have been used to assess clot resolution and guide treatment duration. Of note, in certain circumstances, no suppurative source will be found and patients will be treated for presumed pylephlebitis, given the presence of portal vein thrombi and clinical/laboratory evidence of infection.7
Early treatment of pylephlebitis is essential given the high incidence of mortality when treatment is delayed. Many patients will present in a distressed state, with approximately 20% presenting in sepsis.1 Fluid resuscitation, broad-spectrum antibiotics and eradication of the suppurative source are the mainstays of treatment. Antibiotic coverage should be targeted towards gram negative and anaerobic organisms until culture and sensitivity results are available. Antibiotic coverage for 4 to 6 weeks is generally advised, or until complete resolution of the thrombus is confirmed by ultrasound or CT.7–9
The use of anticoagulation in cases of pylephlebitis continues to be controversial. A small retrospective case report by Baril et al is generally cited in the recommendation of anticoagulation. This study concluded that anticoagulation was indicated for those patients with documented coagulation disorders, or in patients with hypercoagulable states, such as cancer or hematological disease. Anticoagulation is given, in theory, to avoid the more devastating complications of portal vein thrombosis, such as portal hypertension and bowel ischemia. Some investigators note, however, that these complications are rare, given that portal vein thrombosis from pylephlebitis is thought to be non-obstructive.1,6 Although there are multiple case studies that recommend for or against anticoagulation, no study to date clearly demonstrates an advantage in terms of clot resolution or mortality.
In conclusion, we would like to stress the importance of early diagnosis and treatment in cases of pylephlebitis. In the case of our patient, the diagnosis was elusive and delayed until we obtained definitive imaging. Furthermore, even after the diagnosis was made through CT, the primary and consulting teams either missed or dismissed this potentially fatal disease. Luckily, however, appropriate antibiotics were instituted and the suppurative source was removed before further complications arose. Likely, this missed diagnosis was due to a general lack of awareness and understanding of pylephlebitis. Although this is a rare disease, the high mortality that accompanies it necessitates that we keep it in our differential when examining an ED patient with poorly differentiated abdominal pain and fever.
Footnotes
Address for Correspondence: Christopher J. Coyne, MD. LAC+USC Health Network, Department of Emergency Medicine, 1200 N. State Street, Los Angeles, CA 90033. Email: coynec@usc.edu.
Submission history: Revision received November 25, 2012; Submitted December 29, 2012; Accepted January 22, 2013
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1 Chirinos JA, Garcia J, Alcaide ML Septic thrombophlebitis, diagnosis and management. Am J Cardiovasc Drugs. 2006; 6:9-14
2 Rea JD, Jundt JP Pylephlebitis: keep it in your differential diagnosis. Am J Surg. 2010; 200:e69-e71
3 Chang YS, Min SY, Joo SH Septic thrombophlebitis of the porto-mesenteric veins as a complication of acute appendicitis. World J Gastroenterol. 2008; 14:4580-4582
4 Chang TN, Tang L, Keller K Pylephlebitis, portal-mesenteric thrombosis, and multiple liver abscesses owing to perforated appendicitis. J Pediatr Surg. 2001; 36:19-21
5 Baril N, Wren S, Radin R The role of anticoagulation in pylephlebitis. Am J Surg. 1996; 172:449-453
6 Singh P, Yadav N, Visvalingam V Pylephlebitis – diagnosis and management. Am J Gastroenterol. 2001; 96:1312-1313
7 Bogue C, Leahy T, Rea DJ Idiopathic suppurative pylephlebitis: interventional radiological diagnosis and management. Cardiovasc Intervent Radiol. 2009; 32:1304-1307
8 Bleeker-Rovers CP, Jager G, Tack CJ F-18-fluodeoxyglucose positron emission tomography leading to a diagnosis of septic thrombophlebitis of the portal vein: description of a case history and review of the literature. J Intern Med. 2004; 255:419-423
9 Nishimori H, Ezoe E, Ura H Septic thrombophlebitis of the postal and superior mesenteric veins as a complication of appendicitis: report of a case. Surg Today. 2004; 34:173-176


