| Author | Affiliation |
|---|---|
| Matthew L. Mitchell, MD | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Elif Yucebey, MD | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Mitchell R. Weaver, MD | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| A. Kathrin Jaehne, MD | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Emanuel P. Rivers, MD | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
Introduction
Case report
Discussion
Conclusion
ABSTRACT
A 50-year-old man presented to the emergency department (ED) with acute, bilateral lower extremity weakness and loss of sensation, as well as absent pulses bilaterally. Computed tomography angiography showed complete occlusion of the aorta below the inferior mesenteric artery, extending to the iliac bifurcations. Echocardiographic findings showed severe systolic dysfunction (ejection fraction of 15%) and cryptic cardiogenic shock in spite of stable vital signs. Prior to early operative intervention, an early goal-oriented hemodynamic strategy of shock management resulted in the resolution of motor and sensory deficits.After definitive surgical intervention, the patient was discharged neurologically intact. Acute aortic occlusion is frequently accompanied by myocardial dysfunction, which can be from mild to severe. The most severe form can even occur with normal vital signs or occult cardiogenic shock. Early detection and goal-directed preoperative hemodynamic optimization, along with surgical intervention in the ED, is required to optimize outcomes.
INTRODUCTION
Sudden bilateral lower extremity weakness can be the cardinal sign of a complete occlusion of the abdominal aorta. Multiple case reports reveal that myocardial dysfunction leading to global tissue hypoperfusion and shock frequently accompanies this disorder, which is associated with a mortality of 38%.1–19 Therefore, early recognition and therapeutic intervention, aimed at the restoration of tissue perfusion using a goal-directed approach, is paramount to maximize outcomes.
We report the case of a patient presenting with sudden bilateral lower extremity weakness and loss of pulses due to a complete occlusion of the infra-renal abdominal aorta, extending in the bilateral iliac arteries complicated by cardiogenic shock. Early recognition and treatment of these conditions in the emergency department (ED) before surgery can significantly improve survival.
CASE REPORT
A 50-year-old man presented to the ED after sudden onset of bilateral lower extremity weakness. The patient developed lower back pain and tingling in both lower extremities which progressed to complete paralysis within two hours after onset of the symptoms.
His medical history was significant for diet-controlled diabetes mellitus, systolic heart failure, hypertension, prior myocardial infarction, stroke, and prostate cancer. His medications included furosemide, digoxin, carvedilol, lisinopril, clopidogrel, and aspirin. He received hormonal therapy for his prostate cancer within the last year. His social history included 1 pack per day of cigarettes and a remote history of intravenous drug abuse.
At ED arrival, he was found to have a blood pressure of 127/47 mmHg (mean arterial pressure [MAP] of 74 mmHg). Upon physical exam, the patient was alert and oriented to self, time and place. Strength in lower extremities was 0/5 bilaterally, with no sensation from the distal one third of thigh and below bilaterally. Cardiovascular exam revealed regular rate and rhythm, S1, S2, and no pedal edema. However, femoral, dorsalis pedis and posterior tibialis pulses were absent bilaterally, in addition to poor rectal tone. There was mottling over the knees bilaterally, and they were cool to touch.
Laboratory work-up revealed moderately elevated blood urea nitrogen (BUN) and creatinine, 50 and 2.5 mg/dL respectively, a creatine phosphokinase of 323 IU/L, a pH of 7.50 and a troponin of 0.7 mg/mL. Because of the physical exam findings of decreased perfusion and new onset renal failure, a serum lactate was ordered and found to be elevated to 5.6 mmol/L.
The history of prostate cancer led to the inclusion of acute spinal cord compression in the differential diagnosis. Consequently, contrast computed tomography (CT) of brain, thoracic and lumbar spine were ordered, all of which were unremarkable. Due to the absence of pulses in the lower extremities, additional CT angiogram was ordered, which showed a complete occlusion of the distal aorta below the inferior mesenteric artery, extending to the common iliac bifurcations (Figure). To rule out a cardiac source for the thrombus, an echocardiogram was performed and showed an ejection fraction (EF) of 15 %, indicating severe systolic dysfunction. Clinical and laboratory evidence of global tissue hypoperfusion (shock) led to the conclusion that the patient was in need of intensive or invasive hemodynamic monitoring to guide his resuscitation.
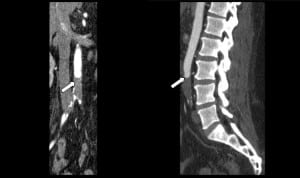
Computed tomography angiography of the thorax and abdomen shows a complete thrombosis of the distal aorta below the inferior mesenteric artery, extending to the common iliac bifurcations.
Paralleling vascular surgery consultation, a central venous catheter was placed and a goal-directed hemodynamic optimization was performed before the necessary surgical intervention. This included optimization of preload, afterload and contractility to increase systemic oxygen delivery to meet demands and eradicate global tissue hypoperfusion. This was achieved with the use of the central venous pressure, (CVP), MAP, ScvO2 and clearance of lactate levels. During the 3 hours of ED stay prior to surgical intervention, Dobutamine was started at 2.5 mcg/kg/min and raised to 5 mcg/kg/min by the second hour. During this time, CVP decreased from 28 to12 cmH2O and ScvO2 increased from 48 to 74 %, while lactate levels decreased from 5.2 to 1.0 mmol/L. During this period MAP was maintained between 75–88 mmHg, and the heart rate ranged from 72 to 81 beats per minute. These findings indicated the initial presence of global tissue hypoxia and its resolution during the goal-directed resuscitation. Severe global tissue hypoxia was present with normal vital signs.
After improvement in hemodynamic status, he underwent an open aorto-ilaic embolectomy and bilateral lower extremity 4-compartment fasciotomies to prevent the expected reperfusion injury and compartment syndrome. The patient was transferred to the surgical intensive care unit after the procedure for continued monitoring, resuscitation and systemic anticoagulation with heparin.
He continued to improve post-operatively and was discharged on anticoagulation. At follow-up evaluation in the vascular surgery clinic, 13 days after hospital discharge, the patient reported full return of lower extremity sensation and was able to ambulate without assistance.
DISCUSSION
Acute aortic occlusion is an infrequent surgical emergency, but should be considered as part of the differential diagnosis in the ED in patients presenting with bilateral loss of motor function, sensation and pulses.2–4,6,10 The differential in this case also includes spinal cord compression from prostate cancer or a spinal abscess (history of intravenous drug abuse).1,4 In this case, the physical exam findings of the bilateral absent pulses in the lower extremities led to the suspicion of a vascular cause.
An important clinical caveat in this case relates to the early detection of global tissue hypoperfusion as a paralleling insult to the physical examination. These findings can be occult (with normal vital signs), especially in patients with pre-existing myocardial disease. Thus, the additional findings of mottling, acute renal failure, lactate elevation and severe systolic dysfunction by echocardiogram suggests global tissue hypoperfusion and necessitates early and aggressive hemodynamic optimization.1,4
In a review of the literature, a common feature is cardiac dysfunction, which can be present as a result of the acute process or exacerbated from pre-existing disease, such as congestive heart failure (CHF).2–4,10 Bell et al2 noted that this “unstable cardiac output” resulted in a failure of the surgical efforts to restore circulation, and ultimately led to an adverse outcome of 100% mortality (6/6 patients died). Dossa et al4 suggested that the decrease in mortality from 40% to 24% observed over the last 40 years might be related to improved perioperative recognition and hemodynamic optimization. Pre-operative cardiac optimization improves the tolerance to a sustained increase in cardiac afterload following the acute aortic occlusion, which further stresses an already compromised myocardium.4 Babu et al1 noted similar findings as baseline mortality increased from 52 % to 85% when pre-existing myocardial dysfunction accompanied this disorder.
Large case series reported by Babu and Dossa1,4 describe the importance of perioperative management with not only systemic anticoagulation and hydration, but also a comprehensive optimization of cardiac function for successful outcomes. Appropriate assessment of cardiac dysfunction in these case series was only possible with invasive monitoring, such as pulmonary and radial artery catheterization. The importance of perioperative restoration of cardiac function is further highlighted by the fact that patients, who did not normalize their left ventricular function (LVF), had worse outcomes. In these studies, 83% of patients with compromised LVF died compared to 23% with improved LVF.1
The compromised cardiac function in patients with acute aortic occlusion, if not recognized and addressed early, can progress in these patients to cardiogenic shock. These findings of cardiac decompensation are observed not only in acute aortic occlusion, but also in chronic occlusions as described by Danto3 in 1997, where the slowly developing chronic aortic occlusion was associated with the cardiac decompensation in a majority of cases with a reported mortality of 25% in the study’s 9 patients.
Patients with known CHF present frequently to the ED with exacerbation of their CHF20 as a medical emergency. These patients can present with normal vital signs, such as MAP and heart rate, as in this presented case. Clinicians confronted with such patients might underestimate the illness severity and the developing cardiogenic shock. As highlighted by Ander, several reports show that in patients with decompensated CHF, global tissue hypoxia secondary to inadequate systemic oxygen delivery (DO2) can exist with normal vital signs.20 Ander calls this occult cardiogenic shock, mandating that once these patients are identified, a targeted optimization of hemodynamic parameters should include lactate, CVP and ScvO2 to improve morbidity and mortality.
The present case of a combination of the acute aortic occlusion with a possible further acute decompensation of the cardiac function leading to cardiogenic shock needs to be addressed by a structured approach to achieve an optimal outcome.
CONCLUSION
This case and the review of the literature highlight that acute aortic occlusion represents a combination of acute neurologic, vascular and hemodynamic emergencies. The combination of a structural lesion causing acute occlusive ischemia is frequently confounded by a decrease in systemic oxygen delivery (DO2), secondary to decreased myocardial function. This myocardial dysfunction can range from mild to the most severe degree of illness severity (cardiogenic shock). Cardiogenic shock can occur with normal vital signs. It has been shown that addressing occult tissue hypoperfusion is associated with decreased morbidity and mortality.20 This highlights the need for emergency hemodynamic recognition and treatment of shock while employing a surgical remediation to this disease process. While the diagnosis of aortic occlusion was notable on CT in the reported case, the underlying occult shock state could have gone unrecognized, possibly leading to increased morbidity and mortality.
Footnotes
Address for Correspondence: Matthew Mitchell, MD, Henry Ford Hospital, Department of Emergency Medicine, 2799 West Grand Boulevard, CFP-258, Detroit, MI 48202. Email: mmitche5@hfhs.org.
Submission history: Revision received August 4, 2012; Submitted January 28, 2013; Accepted February 15, 2013
Conflicts of Interest : By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1 Babu SC, Shah PM, Nitahara J Acute aortic occlusion–factors that influence outcome. J Vasc Surg. 1995; 21:567-572
2 Bell JW Acute thrombosis of the subrenal abdominal aorta. Arch Surg. 1967; 95:681-684
3 Danto LA, Fry WJ, Kraft RO Acute aortic thrombosis. Arch Surg. 1972; 104:569-572
4 Dossa CD, Shepard AD, Reddy DJ Acute aortic occlusion. A 40-year experience. Arch Surg. 1994; 129:603-607
5 Johnson JM, Gaspar MR, Movius HJ Sudden complete thrombosis of aortic and iliac aneurysms. Arch Surg. 1974; 108:792-794
6 Kraev AI, Giashuddin S, Omerovic V Acute aortic occlusion from a Candida fungus ball. J Vasc Surg. 2011; 54:1475-1477
7 Leather RP, Shah D, Goldman M Nonresective treatment of abdominal aortic aneurysms. Use of acute thrombosis and axillofemoral bypass. Arch Surg. 1979; 114:1402-1408
8 Mack L, Forbes TL, Harris KA Acute aortic thrombosis following incorrect application of the Heimlich maneuver. Ann Vasc Surg. 2002; 16:130-133
9 Poiree S, Monnier-Cholley L, Tubiana JM Acute abdominal aortic thrombosis in cancer patients. Abdom Imaging. 2004; 29:511-513
10 Surowiec SM, Isiklar H, Sreeram S Acute occlusion of the abdominal aorta. Am J Surg. 1998; 176:193-197
11 Yamamoto H, Yamamoto F, Tanaka F Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011; 17:422-427
12 Bradbury AW, Stonebridge PA, John TG Acute thrombosis of the non-aneurysmal abdominal aorta. Eur J Vasc Surg. 1993; 7:320-323
13 Corson JD, Brewster DC, Darling RC The surgical management of infrarenal aortic occlusion. Surg Gynecol Obstet. 1982; 155:369-372
14 Drager SB, Riles TS, Imparato AM Management of acute aortic occlusion. Am J Surg Aug. 1979; 138:293-295
15 Lee WA Acute aortic occlusion from a cardiac embolus. J Vasc Surg. 2003; 38:197
16 Littooy FN, Baker WH Acute aortic occlusion–a multifaceted catastrophe. J Vasc Surg. 1986; 4:211-216
17 Meagher AP, Lord RS, Graham AR Acute aortic occlusion presenting with lower limb paralysis. J Cardiovasc Surg (Torino). 1991; 32:643-647
18 Tapper SS, Jenkins JM, Edwards WH Juxtarenal aortic occlusion. Ann Surg. 1992; 215:443-449
19 Shapiro ME, Rodvien R, Bauer KA Acute aortic thrombosis in antithrombin III deficiency. JAMA. 1981; 245:1759-1761
20 Ander DS, Jaggi M, Rivers E Undetected cardiogenic shock in patients with congestive heart failure presenting to the emergency department. Am J Cardiol. 1998; 82:888-891


