| Author | Affiliation |
|---|---|
| Thomas Pero, MD | Texas A&M University, Christus Spohn Memorial Hospital Emergency Department |
| John Herrick, DO | Texas A&M University, Christus Spohn Memorial Hospital Emergency Department |
ABSTRACT
A 42-year-old male presented to the emergency department with pain and swelling of his distal right wrist. Bedside ultrasound placed over the swelling revealed a pseudoaneurysm of the radial artery. The patient received percutaneous thrombin injection of the aneurysm sac followed by direct ultrasound compression therapy of the pseudoaneurysm neck, resulting in thrombosis of the sac. The use of bedside ultrasound by the emergency physician led to appropriate care and proper disposition for definitive management.
INTRODUCTION
Pseudoaneurysms of the radial artery are uncommon. The emergency medicine literature has never reported penetrating trauma as a cause. We discuss a case in which a patient developed a pseudoaneurysm of the radial artery following a penetrating stab wound to the volar aspect of his right wrist. We suggest that physical examination of a pseudoaneurysm is unreliable, and the use of a bedside ultrasound machine in the emergency department (ED) is justified and diagnostic. We also introduce several modalities to repair a pseudoaneurysm to aid the physician in proper disposition for definitive management.
CASE
A 42-year-old male patient presented to a tertiary care academic medical center ED complaining of pain and swelling to his right distal radius. Twenty-six days prior to his current visit, the patient had suffered an accidental self-inflicted stab wound to the volar aspect of his right wrist while skinning deer. During his initial visit at another ED following his laceration, no artery or nerve damage was noted, and the patient was discharged home on oral antibiotics following primary closure of the linear laceration.
On presentation to our ED, the patient stated that he experienced continuous discomfort since the trauma but has now noticed increased pain and swelling on his right volar wrist. The patient now complained of paresthesias to his first, second and third digits on the dorsal aspect. The patient denied fevers, chills, or any other constitutional symptoms that would suggest a possible osteomyelitis or other disease process.
Physical examination revealed a prominent swelling over the volar aspect of his right distal wrist but no overlying erythema (Figure 1A). Palpation of the swelling revealed a discrete pulsatile mass 4cm by 3cm. Distal to the swelling, the radial artery pulse could be palpated deeper and Allen’s test was negative for arterial insufficiency to the palm.
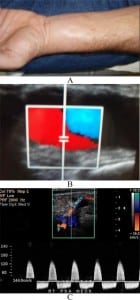
Using our bedside ultrasound machine, a linear vascular probe placed directly over the mass revealed a classic “yin-yang” pattern of turbulent blood flow directly over the radial artery (Figure 1B). This classic swirling pattern of blood flow has been characterized to be highly suggestive of arterial pseudoaneurysms.1
Subsequently, the patient was admitted to the hospital where the interventional radiologist performed ultrasound-guided percutaneous thrombin injection of the sac with 200 units of thrombin, obtaining complete thrombosis of the pseudoaneurysm. On the first post-procedural day, a repeat ultrasound visualized recanalization of the pseudoaneurysm. The patient then underwent ultrasound compression therapy for 30 minutes at the neck of the pseudoaneurysm, successfully obtaining complete thrombosis. Afterwards, the patient’s paresthesias had resolved, and serial neurovascular examinations and ultrasounds of the area revealed persistent thrombosis of the pseudoaneurysm sac. The patient was discharged without further complications. Following up on the patient six months later confirmed resolution of the pseudoaneurysm, and neurovascular status of his hand remained intact.
DISCUSSION
An arterial pseudoaneurysm can be caused by trauma, usually penetrating, of a native vessel. Although the detection of such complications may result within hours from the time of insult, they may occur one to several months afterwards.2
There has been no documentation in the emergency medicine literature regarding formation of arterial pseudoaneurysms after penetrating trauma. The trauma literature has described one case where a pseudoaneurysm formed after laceration from a piece of glass.2 There have been documented cases of pseudoaneurysm formation following vascular access into arteriovenous fistulae, and Komorowska et al.3 have noted 16 cases of radial artery pseudoaneurysms following infected catheters in the distal radial artery.
Due to its rarity in clinical practice, detection of a pseudoaneurysm in the ED is difficult. Depending on the size of the aneurysm sac, a mass might not be palpated leaving pain as the only symptom. If a mass can be palpated, it may be pulsatile, but it may not have a thrill. Since physical examination is unreliable in diagnosing pseudoaneurysms, bedside ultrasound is necessary and is the most useful adjunct in the diagnosis of a pseudoaneurysm. Due to the high number of arterial cannulations conducted in intensive care units, bedside ultrasound already is utilized in the ICU to assess for pseudoaneurysms.4
There are three characteristic features of pseudoaneurysms on ultrasound: the presence of expansile pulsatility, detection of turbulent flow that appears as a classic “yin-yang” sign, and a hematoma with variable echogenicity. The variable echogenicity may represent separate episodes of bleeding and rebleeding.4 Although not demonstrated in our case report, identification of a “to-and-fro” spectral waveform within the neck is considered pathognomonic for a pseudoaneurysm1 (Figure 1C).
With physical examination of a pseudoaneurysm, Allen’s test is generally negative and arterial pulses are usually palpated distal to the mass. Morbidity can be severe and is related to distal embolization from microemboli, venous compression or even frank rupture.1,2 For this reason, it is important for the ED physician to correctly diagnose pseudoaneurysms and provide appropriate definitive care. Older literature stated that arterial aneurysms of the upper extremity should be treated surgically,2 but current research suggests other modalities of correction.
Ultrasound compression therapy involves direct ultrasound-guided compression of a pseudoaneurysm to obstruct the inflow tract of blood. The stasis of blood promotes coagulation causing occlusion and resolution of the pseudoaneurysm. Unfortunately, compression must be maintained for over an hour to obtain occlusion – sometimes longer for anticoagulated patients. Patients can experience extreme discomfort from the prolonged compression. Moreover, the failure rate is significantly high, and surgical intervention may need to be considered if complete occlusion is not achieved. Manghat et al.5 present a case where a radial artery pseudoaneurysm followed cannulation of the radial artery during coronary catheterization. Direct compression of the artery did not achieve occlusion and surgical ligation was subsequently necessary. On the other hand, Witz et al.6 have studied three case reports of patients who developed pseudoaneurysms following vascular access into arteriovenous fistulae in hemodialysis-dependent patients. Patients were all treated with ultrasound compression therapy with complete occlusion of the sac, and no further therapy was needed.
Another option for occlusion of pseudoaneurysms involves ultrasound-guided thrombin injection into the sac. Contrary to compression therapy which may take over an hour, introduction of thrombin starts clot formation instantaneously, but the procedure is not without risks. If thrombin escapes the pseudoaneurysm, a clot can propagate into the affected artery, resulting in embolization or thrombosis of peripheral branch vessels. This may cause extremity paresthesias, pain, or frank necrosis with cases of limb loss being reported.7 D’Achille et al.8 describe a case where thrombin injection led to distal skin changes suggesting embolization of a vessel more distally.
Failure to occlude pseudoaneurysms with thrombin injection occurs, but at a lower rate as compared to ultrasound compression therapy. Corso et al.9 describe a case where two subsequent attempts to occlude a false aneurysm with different concentration of thrombin failed, and Komorowska3 reports a case where thrombin failed occlusion thereby requiring surgical ligation. In our case study, our patient recanalized his pseudoaneurysm after a failed attempt at thrombin injection, but he did manage to obtain complete occlusion after direct compression therapy for 30 minutes the following post-operative day. The combination of both thrombin injection while performing direct compression of the vascular neck with the ultrasound vascular probe, as proposed by Pozniak,7 may increase the effectiveness of therapy.
CONCLUSION
Pseudoaneurysms following arterial injuries are rare occurrences, but they have been described in the literature following vascular access attempts to arteriovenous fistulae, catheterization of arteries, arterial blood gas analysis, and other invasive procedures. This is the first case in the emergency medicine literature to report a pseudoaneurysm following penetrating trauma. This case demonstrates that bedside ultrasound supplements careful history-taking and physical examination in making this difficult diagnosis in the ED.
Footnotes
We would like to acknowledge Julie Gorchynski, MD for her support and guidance.
Supervising Section Editor: Bharath Chakravarthy, MD, MPH
Submission history: Submitted April 17, 2008; Revision Received August 21, 2008; Accepted October 03, 2008.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Thomas Pero, MD. Christus Spohn Memorial Emergency Medicine Residency Program, Texas A&M, 2606 Hospital Boulevard, Corpus Christi, Texas 78405
Email: perothomas@hotmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Rozen G, Samuels D, Blank A. The to and fro sign: the hallmark of pseudoaneurysms.IMAJ. 2001;3:781–782. [PubMed]
2. Kerr C, Duffey T. Traumatic false aneurysm of the radial artery. J Trauma.1988;28:1603–1604. [PubMed]
3. Komorowska-Timek E, Teruya T, Abou-Zamzam A, Papa D, Ballard J. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surgery. 2004;29:936–942.
4. Bouch D, Hall A. Ultrasound diagnosis of a false radial artery aneurysm in ICU.Anaesthesia. 2006;61:1018. [PubMed]
5. Manghat N, Ellwood F, Roobottom C. Radial artery pseudoaneurysm post-cardiac catheterization: Imaging with multidetector row CT peripheral angiography. Heart.2006;9:872. [PMC free article] [PubMed]
6. Witz M, Werner M, Bernheim J, Shnaker A, Lehmann J, Korzets Z. Ultrasound-guided compression repair of pseudoaneurysms complicating a forearm dialysis arteriovenous fistula. Nephrology Dialysis Transplant. 2000;15:1453–1454.
7. Pozniak M, Mitchell C, Ledwidge M. Radial artery pseudoaneurysm: a maneuver to decrease the risk of thrombin therapy. J Ultrasound Medicine. 2005;24:119–122.
8. D’Achille A, Sebben R, Davies R. Percutaneous ultrasound- guided thrombin injection for coagulation of post-traumatic pseudoaneurysms. Australian Radiology. 2001;45:218–221.
9. Corso R, Rampoli A, Vercelli R, Leni D, Vanzulli A. Percutaneous repair of radial artery pseudoaneurysm in a hemodialysis patient using sonographically guided thrombin injection. Cardiovascular and Interventional Radiology. 2006;29:130–132. [PubMed]


