| Author | Affiliation |
|---|---|
| Matthew Hanson, MD | University of Illinois College of Medicine, Department of Surgery, Division of Emergency Medicine, Peoria, Illinois |
| John William Hafner, MD, MPH | University of Illinois College of Medicine, Department of Surgery, Division of Emergency Medicine, Peoria, Illinois |
INTRODUCTION
There are an estimated 5,000–15,000 caustic injuries resulting from ingestion per year in the United States, with bimodal peaks of incidence at <5 and between 20–30 years of age.1,2 Most of these ingestions represent alkali exposures; however, in developing countries, acids are more readily available and result in more injuries.1–3 The source of caustic exposure is commonly from household chemicals.3–7
We report a case of a caustic exposure presenting to the emergency department (ED) from the improper use of a food product. The ingested substance in our case was an alkali solution used to heat the product. OnTech® Hillside made several self-heating food product canisters, such as coffee and soup containers. These food canisters were marketed as a means for commuters, sports enthusiasts, and other people with no readily available heating source to have hot soups and drinks.8 The top compartment of the can contains the food product and the bottom compartment has a calcium oxide heating element and a small bag of water. The underside of the bottom compartment has a peel-off metal lid concealing a button. Pushing down upon the button releases the water and activates the calcium oxide heating element, producing calcium hydroxide and heat. The 2 compartments remain separate, allowing the food compartment to be heated without mixing with the calcium hydroxide. The outside of the can has a small heat sensitive label that changes color when the product is at the proper temperature for consumption. After the ideal temperature is reached, the top of the container can be opened and the product can be consumed (Figure).
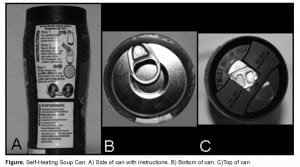
CASE PRESENTATION
The patient is a 54-year-old male who opened a can of OnTech® Hillside tomato soup one morning after consuming a large, but unquantified amount of alcoholic beverages. The patient says he opened the top food-containing compartment of the canister and poured the soup into a bowl. He subsequently cut open the bottom calcium oxide containing compartment of the canister with a pocket knife and combined the powdered heating element with the soup. Upon consuming the mixture, he stated he began feeling an intense burning sensation in the back of his throat. It was at that time he also noted that the mixture was getting hard “like plaster.” He immediately drank a can of beer in an attempt to soothe the burning sensation.
About 45 minutes after the ingestion he reported to the ED complaining of pain with swallowing that radiated into his chest. He denied drooling, hoarseness, dyspnea, abdominal pain, nausea, vomiting, diarrhea, or bloody stools. He also denied any recent illness or history of odynophagia or chest pain. He had medical history significant for hypertension, chronic obstructive pulmonary disease, gastroesophageal reflux disease (GERD), and anxiety. His medications included olanzapine, lisinopril, lorazepam, omeprazole, verapamil, fluoxetine, and zolpidem. He stated that he consumes an unquantified amount of alcohol daily, smokes occasional marijuana, and is a former tobacco smoker. His review of systems was negative except for the presenting complaints.
On physical exam the patient appeared uncomfortable, but was in no acute respiratory distress. His breath smelled of alcohol, but he was alert, oriented, and appeared clinically sober. He was acting and conversing appropriately with good insight. His presenting vital signs were a blood pressure of 152/74 mm Hg, a pulse of 92 beats per minute, respirations of 18 breaths per minute, an oral temperature of 98.20 F, and a room air pulse oximetry reading of 95%. He showed no external signs of trauma and was not drooling. He was noted to have mild erythema in the posterior oropharynx, with no edema, blistering or exudate. He was able to swallow water, but experienced severe pain doing so. His neck was supple with no jugular venous distention, his lungs were clear to auscultation, and his cardiac exam revealed normal heart tones without murmurs. His abdominal examination revealed normal bowel sounds, soft, nontender, no rebound or guarding, no organomegaly, and his stool was negative for gross or occult blood. On neurological examination he was alert and oriented to person, place and time with no focal deficits.
After the initial history and physical exam, an intravenous (IV) line was placed and his pain was treated with intravenous fentanyl. A search of the product website revealed that the chemical powder contained calcium oxide, which forms calcium hydroxide and heat when mixed with water. This added concern for an alkali burn along with thermal burn. The Statewide Poison Control Center was contacted as it was not immediately clear what comprised the ingested powder. The Statewide Poison Control Center was not familiar with the product, but felt it may have contained an iron and charcoal compound that could produce an exothermic reaction when mixed with water. Their advice was to obtain a serum iron level, and treat the ingestion like a thermal burn.
He was found to have an iron level of 103 mcg/dl, a TIBC of 326 mcg/dl, a ferritin level of 131 ng/ml, a serum alcohol level of 135 mg/dl, a hemoglobin of 14.5 g/dl, a hematocrit of 42.0%, a total white blood cell count of 7, 900 cells/HPF, and platelets of 237,000. A comprehensive metabolic panel was normal except for slightly elevated glucose level of 167 mg/dl.
A chest radiograph was obtained and showed a normal cardiac and mediastinal silhouette, with no pneumomediastinum or abdominal free air. A noncontrast chest computed tomography (CT) showed no evidence of esophageal perforation or abnormality. The patient continued to have worsening pain in posterior pharynx radiating down to the chest, as well as odynophagia not well controlled with intravenous narcotic pain medication. The case was discussed with the oncall gastroenterologist and he was consulted for an emergent esophagogastroduodenoscopy (EGD). During the bedside EGD, the patient was found to have no esophageal perforation and no transmural or deep esophageal injury. Mild posterior oropharyngeal irritation and a non-erosive gastritis was noted, and not believed to be related to the ingestion. The patient improved and was subsequently discharged home with sucralfate for 7 days and gastrointestinal (GI) follow up in 1 week, at which time he was lost to follow up.
DISCUSSION
Generally, adult caustic ingestions are much more serious due to the suicidal intent and the large volume consumed.1,3 Our presented case, as described, is an accidental ingestion of a caustic substance and therefore is not what is typically encountered with an adult caustic ingestion. The typical adult ingestion is a purposeful ingestion with suicidal intent using large consumed volumes.1,3 Children account for about 80% of the accidental caustic ingestions and tend to be less severe due to the smaller volume consumed.1,3
No other cases of caustic ingestion due to improper consumption of self-heating soup were found on a search of Medline, Ovid, or the internet. This case was unique as it is the first documented human ingestion of a self-heating element together with a food product, despite clear labeling instructions for preparing the soup. The can states to flush with “generous amounts of water” in case accidental contact with heating material occurs, but makes no comment about what to do in case of accidental ingestion.
The pH of a caustic substance should be considered after any ingestion. A search of the product website did not reveal a pH of the calcium oxide heating element solution. However, according to a material safety data sheet (MSDS) for calcium oxide, a 1% solution has a pH of 10.9 We were unable to determine what the exact concentration the solution was for this ingestion, but the pH was likely <12, although a determination was never conducted. It was most likely a small amount of the substance, and then diluted shortly after ingestion due to the burning sensation our patient felt, which reduced the concentration and contact time. Of interest, this patient diluted the substance by drinking beer after the exposure. The beer consumption may have also had the benefit of removing any potential solid particles that may not have dissolved into solution from the mucosa.
Knowing the potential complications of caustic ingestions, emergency physicians should be aggressive in diagnostic staging if any adult or child presents with concerning history or findings. Hoarseness or stridor can indicate epiglottic or laryngeal involvement, and an evaluation and management of the upper airway should occur.3 Dysphasia, odynophagia, abdominal pain, substernal chest pain, vomiting, and drooling are other worrisome findings that should prompt imaging and possibly endoscopy.3,6,7 Endoscopy is generally considered safe immediately after caustic injury, but should be avoided 5–15 days after exposure due to mucosal sloughing and lack of collagen deposition during this time period.3 There is conflicting evidence regarding diagnostic staging in pediatric ingestion. Some studies state that an asymptomatic child with accidental ingestion and no objective signs of injury can safely be discharged from the ED without EGD, while other studies recommend laryngoscopy and esophagoscopy 48 hours after all pediatric ingestions.3,6,7 Unfortunately, the lack of symptoms has not been proven to preclude need for emergent endoscopy; therefore, clinical suspicion and the type and amount of the caustic ingestion must also be taken into account.3,6
In this case the pH, volume, concentration, and physical state of the ingested substance were not definitively known. Our patient continued to have worsening symptoms of odynophagia, and had objective erythema of posterior oropharynx. These signs and symptoms, coupled with a lack of established experience with the ingested substance, and the potential for long-term complications, determined our need for the emergent EGD.
There is also conflicting evidence and some controversy about the use of steroids and antibiotics after a caustic ingestion.1,3,5,6 Some anecdotal evidence indicates that sucralfate is beneficial in stricture prevention.3 Acid reflux may worsen a caustic injury, so acid suppression therapy in patients with GERD has been recommended.3 Physicians may want to consider usage in all patients, due to the possibility that acid reflux can result from the injury itself.3 We addressed acid suppression therapy in this case by encouraging compliance with his current PPI therapy, the addition of sucralfate and GI follow up.
CONCLUSION
We presented a case of an unusual caustic ingestion with a benign diagnostic EGD. Despite this patient having oropharyngeal erythema and prolonged odynophagia, only a minor injury was sustained from the ingestion. Given this presentation, future ingestions of this type and quantity are likely to be of low risk. However, this represents only one case report; clinical circumstances should still dictate management strategies.
Footnotes
Supervising Section Editor: Rick A McPheeters, DO
Submission history: Submitted January 08, 2012; Revision received March 26, 2012; Accepted April 02, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.4.11734
[West J Emerg Med. 2012;13(5):426-428.]
Address for Correspondence: John William Hafner, MD, MPH. University of Illinois College of Medicine, Department of Surgery, Division of Emergency Medicine, Peoria, Illinois
Email: jhafner@pol.net
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Gumaste VV, Dave PB. Ingestion of corrosive substances by adults. Am J Gastroenterol.1992;87:1–5. [PubMed]
2. Pace F, Antinori S, Repici A. What is new in esophageal injury (infection, drug-induced, caustic, stricture, perforation)? Curr Opin Gastroenterol. 2009;25(4):372–379.
3. Ramasamy K, Gumaste VV. Corrosive ingestion in adults. J Clin Gastroenterol.2003;37(2):119–124. [PubMed]
4. Lamireau T, Rebouissoux L, Denis D, et al. Accidental caustic ingestion in children: Is endoscopy always mandatory? J Pediatr Gastroenterol Nutr. 2001;33:81–84. [PubMed]
5. Cheng HT, Cheng CL, Lin CH, et al. Caustic ingestion in adults: The role of endoscopic classification in predicting outcome. BMC Gastroenterol. 2008;8:31. [PMC free article][PubMed]
6. Riffat F, Cheng A. Pediatric caustic ingestion: 50 consecutive cases and a review of the literature. Dis Esophagus. 2009;22:89–94. [PubMed]
7. Christesen HB. Prediction of complications following unintentional caustic ingestion in children. Is endoscopy always necessary? Acta Paediatr. 1995;84:1177–82. [PubMed]
8. OnTech® Hillside web site. Available at: http://www.ontech.com/new/index.html. Accessed March 26, 2011.
9. Calcium oxide MSDS. Sciencelab website. Available at:http://www.sciencelab.com/xMSDS-Calcium_oxide-9927480. Accessed March 26, 2011.


