| Author | Affiliation |
|---|---|
| Josh M. Sheridan, MD, MS | Loma Linda University, Department of Emergency Medicine, Loma Linda, CA |
| Dustin Smith, MD | Loma Linda University, Department of Emergency Medicine, Loma Linda, CA |
ABSTRACT
Guillain Barré Syndrome (GBS), although an uncommon diagnosis in the emergency department (ED), usually presents as one of the more common chief complaints—weakness. In this report we present an unusual case of weakness, initially seen in the ED and sent home only to return with worsening symptoms and ultimately found to be GBS.
CASE REPORT
A 50-year-old male presented to the emergency department (ED) with a chief complaint of weakness to the upper and lower extremities, increasing over the four days prior to arrival and described as an overall “heavy” feeling. The patient described a constant, yet gradually increasing weakness over this timeframe. He had no previous similar symptoms. The patient denied numbness, paresthesias, headache, fever, neck stiffness, visual changes, shortness of breath, or pain of any kind. The patient stated he had one week of watery diarrhea that had resolved two weeks prior to the onset of his weakness. The review of systems was negative with the exception of the weakness. Past medical history was positive for hypertension only. There was no pertinent family history. Social history was notable for alcohol intake of two to three beers per day. The patient had never smoked or used illicit drugs. He was married and lived at home with his wife.
The physical exam was normal with the exception of mildly decreased proximal muscle weakness bilaterally (4/5), in both upper and lower extremities. The patient had no difficulty ambulating; however, he did have mild difficulty raising himself from a sitting position. The cranial nerves and cerebellar exam were normal. Reflexes were normal in both upper and lower extremities on repeated exams. A head CT and all labs—including complete blood count, electrolytes, liver function, thyroid-stimulating hormone, and erythrocyte sedimentation rate—were within normal limits. The differential diagnosis included Guillain Barré Syndrome (GBS), but this diagnosis was thought to be less likely based on the normal reflexes and isolated proximal muscle weakness. A neurology consultation was requested. After performing their own history and physical exam they recommended outpatient clinic evaluation for electromyography (EMG). While the etiology of the muscle weakness was unclear, they felt GBS was unlikely.
The patient returned to the ED two days later with a progression of symptoms and was seen by a different physician. He was unable to stand up from a sitting position or ambulate without the assistance of two people. On exam his proximal muscle strength in upper and lower extremities had become notably weaker. In addition, distal muscle weakness was now noted. Repeat CBC and electrolytes were unchanged. The patient was admitted to the neurology service where a lumbar puncture was done that showed a protein level of 456mg/L. An EMG was also done, which showed a demyelination pattern consistent with GBS. The patient was started on intravenous immunoglobulin (IVIg) for five days with minimal immediate benefit and was eventually transferred to rehab for further physical therapy.
DISCUSSION
Acute ascending weakness was first described by Landry in 1859, but the full extent of the disease and its characteristics were described by Guillain, Barré and Strohl in 1916.1 The disease gained international notoriety under the name that remains today, Guillain Barré Syndrome.1 GBS is now the world’s most common cause of acute neuromuscular paralysis.2 GBS affects about 0.4–2.4/100,000 people annually with a bimodal incidence in early and late adulthood, however affecting young adults greater then the elderly.2–4 GBS also has a slightly greater male preponderance with a ratio of 1.25–1.5:1.2,5 GBS usually involves a prodromal event such as an upper respiratory infection, vaccination, surgery, or a gastrointestinal infection. It is thought that up to 88% of those affected by GBS have a prodromal infection.6 The most commonly linked organism with GBS is Campylobacter jejuni, and serology testing has identified the organism in as high as 23% of those with GBS.6 One to six weeks after the prodromal event, the patient will experience a sub-acute ascending peripheral weakness with decreased or absent deep tendon reflexes.5
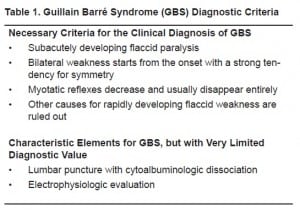
The current diagnostic criteria for GBS are summarized in Table 1 (modified from McGillicuddy). Because there are numerous entities that can cause weakness and sensory deficits, it is imperative that other etiologies be explored prior to the diagnosis of GBS. Possible GBS mimics include poliomyelitis, myasthenia gravis, electrolyte disturbance, botulism, acute myopathy, diphtheria, vasculitis, porphyria, tick paralysis, and toxic neuropathy.2,4
Four subtypes of peripheral neuropathy are classified under the umbrella of GBS; these are acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN), and the Miller Fisher Syndrome (MFS).2,5,7The great majority of those affected with GBS in western countries have the AIDP form where macrophages invade the myelin sheaths directly and cause denuding of the axons impeding conduction along the nerve. In the AMAN and AMSAN variants, the pathology consists of antibodies to ganglioside antigens that lay on the surface of the axon and target the nerve at the nodes of Ranvier and the terminals. Several gangliosides share glycoconjugates similar to those in the bacterial walls of C. jejuni.5,6 The antibodies are formed initially with the C. jejuni bacterial infection and later attack the native axons with the same ganglioside antigens. This is the common link between GBS and C. jejuni. Conduction is altered due to axonal damage rather than myelin destruction in the AMAN and AMSAN variants.6 The last variant is MFS in which weakness starts in the extraocular muscles and can include trunk muscles producing ataxia. This variant usually includes antibodies to a specific antigen known as GQ1b (measured as anti-GQ1b antibody).6 In western countries the Motor-Sensory GBS accounts for 75% of cases, while purely Motor GBS accounts for 20%, and the Miller Fisher Syndrome accounts for about 3%.7
Treatment of GBS includes plasmapheresis or intravenous immunoglobulin (IVIg), as well as respiratory support when needed.2,4–6,8,9 Plasmapheresis and IVIg in GBS have been shown to be equal in efficacy; however, the ease of use of IVIg has made this the treatment of choice.8–10 There was controversy regarding whether a steroid regimen added benefit to therapy, but current recommendations do not support steroid use.9,11 Ventilator support is needed in approximately 25% of GBS cases and in cases with more rapid progression. With treatment, most will have a linear progression of recovery in weeks to months. However, those with a more aggressive onset tend to do more poorly with recovery, and overall 10–20% are left with a disabling motor deficit.5
We report on this patient because he provides a good example of the difficulty that continues to exist in the ED with respect to the diagnosis of GBS. Both the initial emergency physicians (EPs) and the consulting neurologists considered and subsequently discounted GBS as the diagnosis. While presenting with many of the “classic” characteristics of GBS, this patient lacked the two most described characteristics of GBS, distal weakness and decreased reflexes. GBS should be considered in cases of weakness and paresthesia with careful attention to the physical exam. A careful neurologic exam including strength and evaluation of reflexes is imperative when considering GBS in the differential, especially in the ED. In situations where the diagnosis is unclear but where GBS is a part of the differential diagnosis—such as the one presented here—it is important for the EP to give clear discharge instructions with signs to look out for, close follow up as an outpatient, and locations for the patient to follow up. This must be done with the understanding that the patient should return to the ED immediately for any worsening symptoms. GBS is the leading cause of acute neuromuscular paralysis4 and however rare the presentation, we as EPs need to have a keen eye for the presentation characteristics. We have the potential to diagnose and intervene early in the disease progression, both of which benefit the patient’s ultimate outcome.
Footnotes
Supervising Section Editor: Kurt. R. Denninghoff, MD
Submission history: Submitted March 7, 2009; Revision Received June 9, 2009; Accepted June 19, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Josh Sheridan, MD, MS, Department of Emergency Medicine, Loma Linda University Medical Center, 11234 Anderson St, Loma Linda, CA 92354
Email: josh.sheridan@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Bonduelle M. Guillain-Barré syndrome. Arch Neurol. 1998;55(11):1483–4. [PubMed]
2. Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366(9497):1653–66. [PubMed]
3. Hughes R. Campylobacter jejuni in Guillain-Barré syndrome. Lancet Neurol. 2004;3(11):644.[PubMed]
4. McGillicuddy DC, Walker O, Shapiro NI, et al. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390–3. [PubMed]
5. Cosi V, Versino M. Guillain-Barré syndrome. Neurol Sci. 2006;27(Suppl 1):S47–51. [PubMed]
6. Tsang RS. The relationship of Campylobacter jejuni infection and the development of Guillain-Barré syndrome. Curr Opin Infect Dis. 2002;15(3):221–8. [PubMed]
7. Van der Meché FG, Van Doorn PA, Meulstee J, et al. GBS-consensus group of the Dutch Neuromuscular Research Support Centre, Diagnostic and classification criteria for the Guillain-Barré syndrome. Eur Neurol. 2001;45(3):133–9. [PubMed]
8. Stangel M, Pul R. Basic principles of intravenous immunoglobulin (IVIg) treatment. J Neurol.2006;253(Suppl 5):V18–24. [PubMed]
9. Darabi K, Abdel-Wahab O, Dzik WH. Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature.Transfusion. 2006;46(5):741–53. [PubMed]
10. Hughes RA, Raphaël JC, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2006;(1):CD002063. [PubMed]
11. Hughes RA, Swan AV, van Koningsveld R, et al. Corticosteroids for Guillain-Barré syndrome.Cochrane Database Syst Rev. 2006;(2):CD001446. [PubMed]


