| Author | Affiliation |
|---|---|
| Kenneth Deitch, DO | Einstein Medical Center, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Adam Rowden, DO | Einstein Medical Center, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Kathia Damiron, MD | Einstein Medical Center, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Claudia Lares, MD | Einstein Medical Center, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Nino Oqroshidze, MD | Einstein Medical Center, Department of Emergency Medicine, Philadelphia, Pennsylvania |
| Elizabeth Aguilera, MD | Einstein Medical Center, Department of Emergency Medicine, Philadelphia, Pennsylvania |
Introduction
Objective
Materials and methods
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
The incidence of respiratory depression in patients who are chemically sedated in the emergency department (ED) is not well understood. As the drugs used for chemical restraint are respiratory depressants, improving respiratory monitoring practice in the ED may be warranted. The objective of this study is to describe the incidence of respiratory depression in patients chemically sedated for violent behavior and psychomotor agitation in the ED.
Methods
Adult patients who met eligibility criteria with psychomotor agitation and violent behavior who were chemically sedated were eligible. SpO2 and ETCO2 (end-tidal CO2) was recorded and saved every 5 seconds. Demographic data, history of drug or alcohol abuse, medical and psychiatric history, HR and BP every 5 minutes, any physician intervention for hypoxia or respiratory depression, or adverse events were also recorded. We defined respiratory depression as an ETCO2 of ≥50 mmHg, a change of 10% above or below baseline, or a loss of waveform for ≥15 seconds. Hypoxia was defined as a SpO2 of ≤93% for ≥15 seconds.
Results
We enrolled 59 patients, and excluded 9 because of ≥35% data loss. Twenty-eight (28/50) patients developed respiratory depression at least once during their chemical restraint (56%, 95% CI 42–69%); the median number of events was 2 (range 1–6). Twenty-one (21/50) patients had at least one hypoxic event during their chemical restraint (42%, 95% CI 29–55%); the median number of events was 2 (range 1–5). Nineteen (19/21) (90%, 95% CI 71–97%) of the patients that developed hypoxia had a corresponding ETCO2 change. Fifteen (15/19) (79%, 95% CI 56–91%) patients who became hypoxic met criteria for respiratory depression before the onset of hypoxia. The sensitivity of ETCO2 to predict the onset of a hypoxic event was 90.48% (95% CI: 68–98%) and specificity 69% (95% CI: 49–84%). Five patients received respiratory interventions from the healthcare team to improve respiration [Airway repositioning: (2), Verbal stimulation: (3)]. Thirty-seven patients had a history of concurrent drug or alcohol abuse and 24 had a concurrent psychiatric history. None of these patients had a major adverse event.
Conclusion
About half of the patients in this study exhibited respiratory depression. Many of these patients went on to have a hypoxic event, and most of the incidences of hypoxia were preceded by respiratory depression. Few of these events were recognized by their treating physicians.
INTRODUCTION
When non-medical techniques for de-escalation (i.e. verbal de-escalation techniques, show of force, bargaining, and family recruitment) fail for the acutely agitated patient in the emergency department (ED), physical restraint and chemical sedation may be required. Pharmacological therapy is recommended to be used with physical restraint of the acutely agitated patient to reduce the risk of complications such as hyperthermia, lactic acidosis, and rhabdomyolysis.1–3
The administration of drugs for psychomotor agitation such as benzodiazepines and butryphenones, especially in combinations, may lead to hypoxia.1–3 American College of Emergency Physicians clinical guidelines recommend vital sign assessment as well as pulse oximetry.3 Routine management in our ED is to place all chemically restrained patients on continuous cardiac and pulse oximetry monitoring.
It is likely that patients receiving chemical sedation are also at risk for respiratory depression that may lead a hypoxic event. As an example, we know from procedural sedation literature that most of the patients who develop hypoxia have significant capnographic signs of respiratory depression well before their SpO2 begins to drop.4–6 Patients receiving chemical sedation are often sedated for long periods of time, and the synergistic effects of concomitant drug and or alcohol use may increase their risk of respiratory compromise.7–12
Further, many of these sedation studies illustrate a concerning issue, i.e. that practitioners don’t recognize the clinical signs of respiratory depression in sedated patients until the patient develops hypoxia. In a randomized controlled trial of capnography use during adult ED sedation with propofol, clinicians monitoring patients for respiratory depression and hypoxia using capnography had a significantly lower rate of hypoxic events than did a group of physicians using standard monitoring only (blinded to capnography).13 This illustrates the theoretical need for improved monitoring practice during chemical sedation, as these patients are often sedated for much longer periods of time, and may not have the same level of attention during a busy ED shift as a patient undergoing procedural sedation. Despite this, capnography is not currently recommended or routinely used for monitoring during chemical sedation.
This study evaluated capnography in addition to standard monitoring to determine the incidence of respiratory depression in a selected population of ED chemically-restrained patients. Since the drugs used in procedural sedation and in chemical restraint have similar respiratory effect profiles, we hypothesized that the incidence of unrecognized respiratory depression is similar as well.
OBJECTIVE
The goal of this study was to determine the incidence of respiratory depression in ED patients with violent behavior and psychomotor agitation who are chemically sedated.
MATERIALS AND METHODS
Study Design
This prospective, observational cohort study was conducted from January 2009 to February 2010. It received institutional review board approval. A waiver of consent was granted secondary to the minimally invasive nature of the study as well as the minimal risks, and the inability to obtain consent from patients beforehand due to their mental status. This study was funded by the ED.
Setting and selection of participants
This study was conducted at Einstein Medical Center, Philadelphia, PA, a Level I trauma center with an annual ED volume of 100,000 patients. Enrollment was done 24 hours a day, 7 days a week. Eligible patients were 18–60 years old or greater and deemed by the treating ED physician to need chemical sedation for violent behavior and psychomotor agitation. We excluded patients if they were pregnant, underage, had a history of dementia, were from a nursing home, had a history of allergies to the sedation agents, or had evidence of recent traumatic injuries.
Data collection and processing
Initial routine management of the violent patient with psychomotor agitation in our ED is to attempt to use non-medical techniques for de-escalation (i.e. verbal de-escalation techniques, show of force, bargaining, and family recruitment). When this fails, a combination of security and ED staff will attempt to physically control the patient until he can be sedated. Vital sign, SpO2, and blood glucose assessment is done before chemical sedation except in the most extreme circumstances. Every attempt is made to get a history from EMS and or family; especially regarding psychiatric history and potential intoxicants. In our ED most of our most violent patients with psychomotor agitation are often acutely intoxicated and often have an underlying history of psychosis.
When an eligible patient was identified, a research associate would report to the bedside to begin data collection. Physical restraint and need for chemical sedation was left to the discretion of the treating physician. Standard protocol in our ED for physical restraint is in the supine position only, with the head of the bed at 30 degrees.
Chemical sedation was not standardized, and was left to physician discretion. However, the most common agents used at our institution are haloperidol and lorazepam. These drugs are both recommended for rapid tranquilization for severe psychomotor agitation with violent behavior.2 Haloperidol is generally recommended as the drug of choice to sedate a violent patient, and rapid sedation is usually achieved using dosages of 2.5 to 10 mg at a time.4 While IV use of Haloperidol is not FDA approved, use in this form is widespread, and is part of routine management in our ED.2 Lorazepam is often used in our institution, as it works well in the violent patient who is intoxicated while efficacious in acute psychosis14 (dosed up to 2 mg IV) to clinical effect. Haloperidol is dosed no more than 5 mg IM or IV at a time, and subsequent doses titrated to clinical effect. The combination of lorazepam with haloperidol has been studied prospectively and has been shown to be superior to either drug alone.8 The use of haloperidol (up to 5 mg) and lorezapam (up to 2 mg) IV may be given and dosages repeated every 30 minutes. This combination (which enjoys popularity as known as the “5 and 2”) allows for synergistic effects with a minimization of major adverse events.
Research associates were responsible for all data collected. Research associates (all non-practicing physicians who had gone through extensive training in procedural sedation, monitoring techniques, Ramsay sedation score, and management for respiratory depression) placed the patient on the research monitor, and were at the bedside during ED monitoring. They collected patient demographic data (age, weight, medical history) from the ED chart. A nasal cannula capable of simultaneously delivering oxygen and measuring expired carbon dioxide was placed on all study patients. The portable monitor (Capnostream 20, Oridion Medical, Needham, MA) recorded SpO2 and ETCO2 (end-tidal CO2) from the patient every 5 seconds with no set alarm for a specified SpO2 or ETCO2. These data were recorded and downloaded into an Excel spreadsheet for further analysis. The healthcare team had real time access to standard monitor with SpO2, respiratory rate, heart rate, and blood pressure at all times. As capnography was not deemed part of routine practice outside of procedural sedation monitoring, the physicians were blinded to that data. The research associates only observed and recorded data; at no time did they communicate patient information or clinical status to the healthcare team. The only patient contact the research associates were allowed was to adjust the nasal cannula and the pulse oximeter probe if they became dislodged. The research associates recorded blood pressure and heart rate at baseline and every 5 minutes, unless there was an intervention, in which case they recorded vital signs every 60 seconds until the healthcare team judged that the patient’s condition had improved. The research associates recorded any physician or nursing intervention to improve respiratory status, as well as any recognition without intervention “check” from a member of the healthcare team. Administration of medications and interventions were electronically time stamped into the monitor for further review. Major adverse events, including use of bag valve ventilation and endotracheal intubation, were recorded. Patients were observed for up to 90 minutes post-medication administration. After 90 minutes, data were no longer collected. Concurrent medications, positive alcohol and urine drug screens were recorded if available.
Data Analysis
After the patient encounter, all device data were transmitted to a database for analysis. We assigned each research subject a sequential number to preserve patient confidentiality. Electronic data from each sedation were downloaded from the monitor into a Microsoft Excel 2000 (Microsoft, Redmond, WA) database and were checked for any discrepancies with handwritten notations taken by the research associates. A printed time evaluation graph of the duration the patient was chemically restrained was then produced
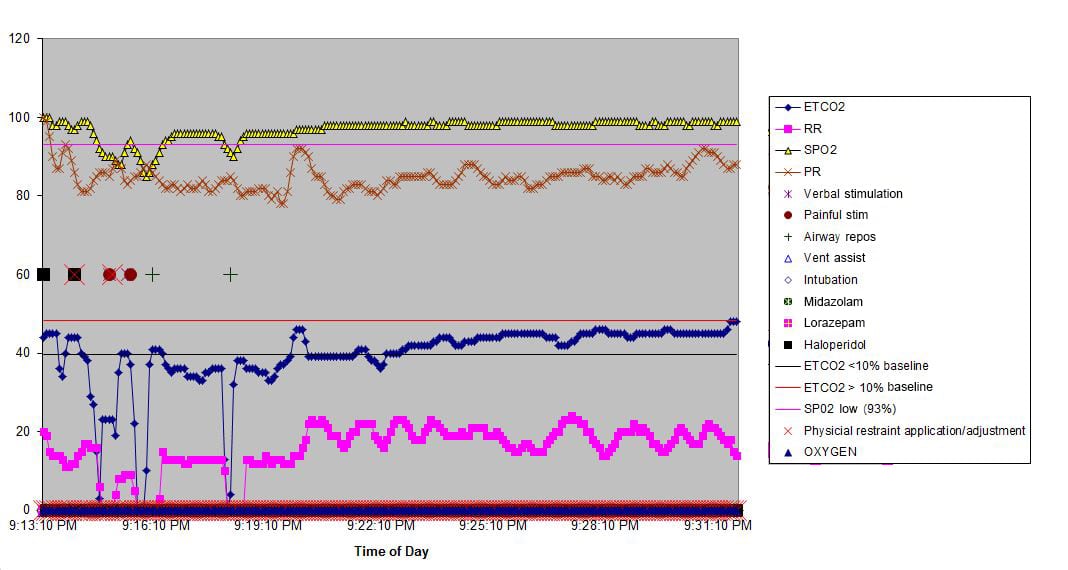
Figure 1
Sample graph from restraint via Capnostream 20.
ETCO2, end-tidal CO2; RR, repiratory rate; SPCO2, skin partial pressure CO2; PR, pulse rate; SPO2, skin partial pressure O2
, with the x axis showing time and the y axis depicting ETCO2, SpO2, respiratory rate, and heart rate. Electronic time stamps were plotted with specific signatures, such as medication administration, physician intervention(s), and adverse events (positive pressure ventilation, endotracheal intubation, or admission to the hospital for a sedation related event.)
Three investigators evaluated each graph to code the presence or absence of hypoxia and respiratory depression. All 3 needed to agree on the disposition of each graph. Hypoxia was defined a priori as a SpO2 level of less than 93% for 15 seconds or greater. Respiratory depression was defined a priori as an ETCO2 level of 50 mm Hg or greater, an absolute increase or decrease from baseline of 10% or greater, or a loss of waveform (for 15 seconds or greater).16–19 There are 2 types of hypoventilation that are pharmacologically induced. Bradypneic hypoventilation (type 1) is characterized by an increased ETCO2 and an increased PaCO2. Respiratory rate is depressed proportionally greater than tidal volume, resulting in bradypnea, an increase in expiratory time, and an increase in ETCO2, graphically represented by a high amplitude and wide capnogram. Bradypneic hypoventilation is commonly observed with opioids or ETOH. Bradypneic hypoventilation (decreased respiratory rate, high amplitude, and wide capnogram) can readily be distinguished from hyperventilation (increased respiratory rate, low amplitude, and narrow capnogram. This type of hypoventilation is associated with sedative hyponotics.20
We disqualified graphs if they had greater than 35% data loss, unless all 3 evaluators agreed that there was unequivocal evidence of hypoxia or respiratory depression. We then compared the data extrapolated from the graphs to the patient’s observed file for final evaluation.
All data was evaluated via SPSS software (IBM, Chicago IL). Descriptive data is reported as means and medians with standard deviations and ranges as appropriate.
RESULTS
Over the 13-month enrollment period, 153 patients were screened and 59 patients enrolled. Eighty patients met exclusion criteria (5 allergic to study drug, 29 trauma patients, 2 pregnant, 9 underage, 7 over age) or did not receive chemical sedation (27). Fourteen patients were missed (research associates could not or did not get to the bedside to enroll), and we excluded 9 because of greater than 35% data loss, leaving 50 patients for analysis. Since this was purely a pilot, observational study, we did not perform a power calculation. Demographic and baseline data for this patient population is reported in Table 1.
Table 1. Patient characteristics with and without respiratory depression and hypoxia.
| Respiratory depression and hypoxiaN=19 | No respiratory depression, no hypoxiaN=20 | |
|---|---|---|
| Median age (years) | 30 (range: 18–57) | 32 (range 18–60) |
| Gender (# of female) | 31% | 35% |
| Median weight (kg) | 79 (range: 52–157) | 78 (range: 57–142) |
| Median Ramsey scores* | 2.6 (range: 1–6) | 2.5 (range 1–6) |
*taken once physician judged the patient to be sedated enough to no longer need initial doses of sedatives
The mean Ramsay sedation score was 2.7 (range 1–4). This was recorded after the treating physician deemed the patient appropriately sedated and additional sedatives were not acutely administered. Twenty-eight (28/50) patients developed respiratory depression at least once during their chemical sedation (56%, 95% CI 42–69%); the median number of events was 2 (range 1–6).
Of the 28 patients who had respiratory depression, 6/28 (21%, 95% CI 10–40%) received a benzodiazepine only, 22/28 (78%, 95% CI 60–90%) received both a benzodiazepine and an anti-psychotic. Of the 22 patients who did not develop hypoxia or respiratory depression, 6/22 (27%, 95% CI 14–48%) received a benzodiazepine only, 16/22 (73%, 95% CI: 52–87%) received both. The mean lorazepam dose (total) was 0.05 mg/Kg; the mean haloperidol dose was 0.07 mg/Kg in both the respiratory depression/hypoxia group and the no respiratory depression/hypoxia group. There were no significant differences in the rate of respiratory depression dependent on if a subject received a benzodiazepine or a combination of drugs.
Twenty-one (21/50) patients had at least 1 hypoxic event during their chemical sedation (42%, 95% CI 29–55%). Of the 21 patients who had a hypoxic event, the median number of events was 2 (range 1–5). Nineteen (19/21) (90%, 95% CI 71–97%) of the patients that developed hypoxia had a corresponding ETCO2 change. Fifteen (15/19) (79%, 95% CI 56–91%) patients who became hypoxic met criteria for respiratory depression before the onset of hypoxia. The median length of time between the onset of an ETCO2 change to the onset of hypoxia was 1.39 minutes (range 15–332 sec).
Significant numbers of patients who were in this study had positive urine drug screens and ETOH levels. As this was an observational study, not all patients enrolled (5/28, 18% of patients who had respiratory depression; 7/22, 31% of patients who did not have respiratory depression) had urine drug screen and or an alcohol level ordered.
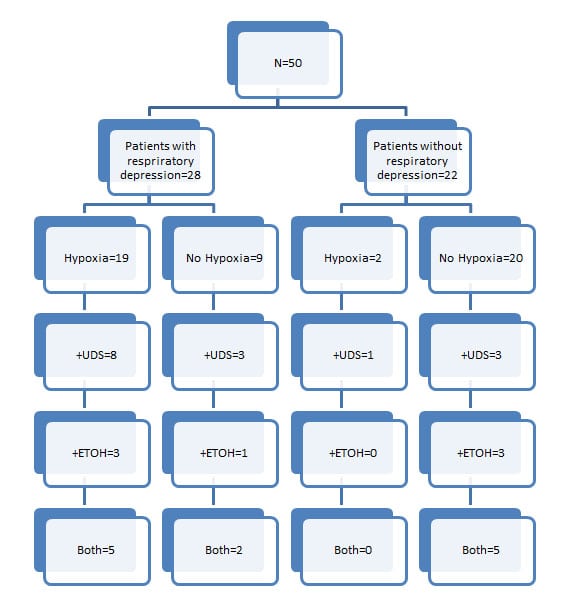
Figure 2 describes the relationships between the patients with and without respiratory depression, and patients with or without hypoxia. It goes on to illustrate patients in each group with the presence or absence of either/or ETOH or a positive urine drug screen. Of note, 16 out of 19 patients with respiratory depression that became hypoxic had a positive ETOH level, a positive urine drug screening, or both (85%, 95% CI 64–95%). Many patients who did not have respiratory depression or hypoxia tested positive for ETOH or illicit drugs (11 out of 20, 55%, 95% CI: 34–74%). As this was an observational study, not all patients (5/28, 18% of patients who had either/or hypoxia and respiratory depression; 7/22, 31% of patients who did not have either hypoxia or respiratory depression) who were enrolled in this study had urine drug screen and or an alcohol level ordered.
The sensitivity of capnographic evidence of respiratory depression as predictive of the onset of a hypoxic event was 90.48% (95% CI: 68–98%) and specificity 69% (95% CI: 49–84%). Five patients received interventions for clinical respiratory depression and hypoxia (Airway repositioning: [2], Verbal stimulation: [3]) Thirty-seven patients had a history of concurrent drug or alcohol abuse, and 24 had a concurrent psychiatric history. None of these patients needed positive pressure ventilation, endotracheal intubation, or admission to the hospital for a sedation related event.
DISCUSSION
Patients who are chemically restrained are given drugs that are respiratory depressants, are often physically restrained, and are often using intoxicants that may potentiate respiratory depression. In this preliminary study, we evaluated the incidence of hypoxia with or without respiratory depression in ED patients receiving chemical sedation (with or without physical restraint) for psychomotor agitation using standard monitoring and capnography. Almost half the patients developed hypoxia and respiratory depression. Many of these patients had multiple episodes of hypoxia and or respiratory depression, and few had any intervention. Healthcare providers in the ED should consider that patients with violent behavior and psychomotor agitation who are given chemical sedation and physical restraint may require continuous electronic vital sign monitoring, including real time SpO2 and ETCO2. We believe that strict monitoring practice of these patients is important; for while it is entirely likely that many patients can tolerate prolonged periods of respiratory depression and hypoxia, predicting which patient will go on to a serious adverse event is beyond the scope of this study.
What is the significance of capnographic evidence of respiratory depression in this population? Most of the patients in this study who developed hypoxia had a corresponding ETCO2 change before the onset of hypoxia. This is consistent with previous studies of the use of capnography to detect respiratory depression during procedural sedation. The mean time between the onset of respiratory depression and the onset of hypoxia as measured via capnography may give a healthcare provider monitoring ETCO2 ample time to intervene to prevent a hypoxic event. Previous work with capnography during ED procedural sedation has shown that clinicians can lower the rate of hypoxic events by using capnography concurrently with standard monitoring techniques. It is conceivable that the same would hold true during chemical sedation.
A few patients had a “false positive” result, i.e. they had respiratory depression without hypoxia 7/28 (25%, 95% CI 17–41%). The incidence of what is defined as “sub-clinical” respiratory depression (capnographic evidence of respiratory depression without hypoxia) has been illustrated in many procedural sedation studies.16–21 The clinical relevance of this is not well understood. Two patients had false negative results, (i.e. developed hypoxia without capnographic evidence of respiratory depression). Despite the false negative results, the sensitivity of capnography to predict a hypoxic event in this study was over 90%, which is quite good for a screening test.
Figure 2
Flow diagram of patients with and without respiratory depression and the association with hypoxia and presence or absence of intoxicants.
ETOH, alcohol; UDS, urine drug screening
This is a preliminary study. A larger study designed to be a head-to-head trial of chemical sedation with blinded capnography versus chemical restraint, with the physicians having real time access to the monitor, is warranted. Capnography during chemical sedation may act as an “early warning” system for the healthcare team to provide an intervention to improve ventilation before the onset of a hypoxic event.
LIMITATIONS
A lack of standardization of sedation protocols may have affected the conclusions drawn from this study. Because many but not all patients had an alcohol or urine drug screen evaluated, it is impossible to derive the relationship between these tests and the incidence of hypoxia and respiratory depression in this study. Many but not all the patients were at some point physically restrained as well; this study was not designed to derive the relationship between physical restraints and respiratory depression. As this was an observational trial, drug dosing was not standardized. It is possible that with standardized dosing there may have been lower levels of respiratory depression and hypoxia. It is possible that suboptimal dosing of haloperidol with lorazepam may have contributed to the high rate of hypoxia and respiratory depression. However, we believe using these drugs in combination to be common practice in emergency medicine.
It is possible that the effect of having a research associate in the room may have biased our data, in that the healthcare team may have responded less or more due to their presence. If a strict protocol for data collection including patient weight had been implemented, more accurate data on the influence of BMI and drug dosing on respiratory depression and hypoxia would have been available.
Our rates of respiratory depression and hypoxia may not be representative of other similar institutions. Other institutions may use other drug regimens or more intensive monitoring practice, and thus their rates of respiratory depression and hypoxia may be lower (or higher) than ours. However, we feel based on our clinical experience that our ED experience with chemical sedation is fairly common among EDs.
A limitation of our conclusions is the lack of any adverse outcomes despite high levels of hypoxia and respiratory depression. A much larger study would have to be conducted to measure that; with current knowledge and technology we are not able to predict which patients will go on to have an adverse outcome. However, we believe that a prudent ED physician would consider that prolonged levels of hypoxia and respiratory depression in a patient with multiple intoxicants and significant chemical sedation is not quality care.
The cause for psychomotor agitation could not be determined in all cases. Because of privacy provisions, we were not able to evaluate previous patient records if the patient had a psychiatric history, thus any data received was gleaned from the treating healthcare team at the time of patient contact. This information may be inaccurate.
Some of these patients may have had some form of obstructive pulmonary disease, which may have pre-disposed them to hypoxia and or respiratory depression. Our “cut-offs” for hypoxia are only relevant for patients at or about sea level; if this study had been repeated at altitude, our tolerances may have changed. However strict hospital guidelines and policy were followed; none of the patients were placed in the prone position and all were seated at 30 degrees or more.
CONCLUSION
Almost half of the patients in this study exhibited respiratory depression; many went on to become hypoxic as well. Most of the incidences of hypoxia were preceded by respiratory depression. Capnography predicted the majority of hypoxic events in this observational study. Few of these events were recognized by their treating physicians. This preliminary study illustrates the need for further research that will help improve early recognition of patients who are chemically restrained who develop respiratory depression and or hypoxia.
Table 2. Patients with hypoxia with and without respiratory depression.
| Patient | Medications given | Urine drug screen result | ETOH level (mg/dL) | SpO2 (lowest) | ETCO2 >50 mmHg | ETCO2 >10% change from baseline | Loss of Waveform | Intervention # |
|---|---|---|---|---|---|---|---|---|
| 1 | Lorazepam 2 mg, Haloperidol 5 mg | THC, opiate, PCP | N/A | 93% (25 sec) | no | yes (85 sec) | no | 2 (physical/verbal stimX2) |
| 2 | Lorazepam 2 mgHaloperidol 5 mg | Cocaine, THC | N/A | 90% (145 sec) | no | no | no | 2 (verbal stim/airway reposition) |
| 3 | Lorazepam 15 mgHaloperidol 5 mg | N/A | N/A | 89% (185 sec) | no | yes (240 sec) | yes (25 sec) | 0 |
| 4 | Lorazepam 4 mgHaloperidol 5 mg | N/A | 146 | 87% (125 sec) | no | yes (205 sec) | yes (45 sec) | 0 |
| 5 | Lorazepam 6 mg | THC | 223 | 86% (75 sec) | no | yes (120 sec) | no | 0 |
| 6 | Lorazepam 4 mg | THC/Benzo | N/A | 91% (35 sec) | no | yes (135 sec) | no | 0 |
| 7 | Lorazepam 2 mgHaloperidol 5 mg | THC/PCP | N/A | 90% (240 sec) | no | yes (310 sec) | no | 0 |
| 8 | Lorazepam 2 mgHaloperidol 10 mg | THC/PCP | N/A | 86% (65 sec) | no | yes (110 sec) | yes (20 sec) | 0 |
| 9 | Lorazepam 4 mgHaloperidol 5 mg | Cocaine | 274 | 89% (25 sec) | yes (205 sec) | yes (250 sec) | yes (35 sec) | 1 (physical/verbal stim) |
| 10 | Lorazepam 2 mgHaloperidol 5 mg | N/A | 81 | 92% (75 sec) | no | yes (110 sec) | yes (45 sec) | 0 |
| 11 | Lorazepam 6 mgHaloperidol 5 mg | N/A | N/A | 90% (45 sec) | no | yes (95 sec) | no | 0 |
| 12 | Lorazepam 4 mgHaloperidol 5 mg | THC, Opiates | 135 | 85% (345 sec) | yes (305 sec) | yes (315 sec) | yes (40 sec) | 0 |
| 13 | Lorazepam 6 mg | THC/PCP | 229 | 90% (135 sec) | yes (45 sec) | yes (160 sec) | yes (15 sec) | 0 |
| 14 | Lorazepam 2 mgHaloperidol 5 mg | THC/PCP | N/A | 91% (50 sec) | no | yes (55 sec) | no | 0 |
| 15 | Lorazepam 4 mg | Cocaine | N/A | 89% (95 sec) | no | yes (125 sec) | no | 0 |
| 16 | Lorazepam 4 mgHaloperidol 5 mg | N/A | N/A | 93% (35 sec) | no | no | no | 0 |
| 17 | Lorazepam 8 mgHaloperidol 5 mg | THC | 117 | 87% (140 sec) | yes (145sec) | yes (150 sec) | yes (25 sec) | 1 (physical/verbal stim, airway reposition) |
| 18 | Lorazepam 2 mgHaloperidol 5 mg | THC/PCP | 203 | 92% (65 sec) | no | yes (130 sec) | no | 0 |
| 19 | Lorazepam 4 mg | THC/PCP | 156 | 91% (50 sec) | no | yes (75 sec) | no | 1 (verbal stim) |
| 20 | Lorazepam 2 mgHaloperidol 10 mg | N/A | N/A | 90% (55 sec) | no | yes (95 sec) | yes (15 sec) | 0 |
| 21 | Lorazepam 6 mg | N/A | N/A | 87% (150 sec) | yes (205 sec) | yes (225 sec) | yes (30 sec) | 0 |
STIM, stimulation; PCP, phencyclidine; THC, tetrahydrocannabinol; ETOH, alchohol; ETCO2, end-tidal CO2
Footnotes
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Kenneth Deitch, DO. Department of Emergency Medicine, Albert Einstein Medical Center, 5501 Old York Road, Korman Building, B-6, Philadelphia, PA 19141. Email: deitchk@einstein.edu. 7 / 2014; 15:430 – 437
Submission history: Revision received July 30, 2013; Submitted November 22, 2013; Accepted February 3, 2014
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1 Coburn VA, Mycyk MB Physical and Chemical Restraints. Emerg Med Clin N Am. 2009; 27:655-667
2 Moore GP, Kao L . The Combative Patient. Rosen’s Emergency Medicine. 2006; 3:2956-71
3 Lukens TW, Wolf SJ, Edlow JA Clinical Policy: Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient. American College of Emergency Medicine. Ann Emerg Med. 2005;
4 Dubin WR, Feld JA Rapid Tranquilzation of the violent patient. AM J Emerg Med. ; 7:1989
5 Currier GW, Trenton A Pharmacologic treatment of psychotic agitation. CNS drugs. 2002; 16:219
6 Binder RL, McNeil DE Emergency psychiatry: Contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatr Serv. ; 50:1999
7 Allen MH, Currier GW, Hughes DH Expert consensus guideline series: Treatment of behavioral emergencies. Postgrad Med. ; :1-88
8 Battaglia J Pharmacologic management of acute agitation. Drugs. 2005; 65:1207-1222
9 Battaglia J, Moss S, Rush J Haloperidol, lorazepam, or both for psychotic agitation? A multi-center, prospective, double-blind, emergency department study. Amer J Emerg Med. 1997; 15:335-340
10 Knott JC, Taylor DM, Castle DJ Randomized clinical trial comparing intravenous midazolam and droperidol for sedation of the acutely agitated patient in the emergency department. Ann Emerg Med. 2006; 47:61-67
11 Wilson MP, MacDonald KS, Vilke GM A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2011;
12 Vilke GM, Wilson MP Agitation: What every emergency physician should know. Emergency Medicine Reports. 2009; 30:233-244
13 Deitch K, Miner J, Chudnofsky CR Does End Tidal CO(2) Monitoring During Emergency Department Procedural Sedation and Analgesia With Propofol Decrease the Incidence of Hypoxic Events? A Randomized, Controlled Trial. Ann Emerg Med. 2010; 55:258-64
14 Citrome L, Volavka J Violent patients in the emergency setting. Psychiatr Clin North Am. ; 22:1999
15 Bellienr TJ Continum of care: Stabilizing the acutely agitated patient. Am J Health Syst Pharm. 2002; 59:s12
16 Burton JH, Harrah JD, Germann CA Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices?. Acad Emerg Med. 2006; 13:500
17 Miner JR, Heegaard W, Plummer D End-tidal carbon dioxide monitoring during procedural sedation. Acad Emerg Med. 2002; 9:275
18 Abramo TJ, Wiebe RA, Scott S Noninvasive capnometry monitoring for respiratory status during pediatric seizures. Crit Care Med. 1997; 25:1242-1246
19 Deitch K, Chudnofsky CR, Dominici P The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized, controlled trial. Ann Emerg Med. 2007; 49:1-8
20 Krauss B, Hess HR Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007; 50:172-81
21 Deitch K, Miner J, Chudnofsky CR Does End Tidal CO2 Monitoring During Emergency Department Procedural Sedation And Analgesia With Propofol Decrease The Incidence Of Hypoxic Events? A Randomized, Controlled Trial. Ann Emerg Med. 2010; 55:258-64