| Author | Affiliation |
|---|---|
| Meena Zareh, BS | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California, Los Angeles, California |
| Andrew Davis, BS | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California, Los Angeles, California |
| Sean Henderson, MD | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California, Los Angeles, California Department of Preventive Medicine, Keck School of Medicine of the University of Southern California, Los Angeles, California |
ABSTRACT
Warfarin, an oral vitamin K antagonist, is used to prevent arterial and venous thromboembolism in patients suffering from a multitude of diseases. In 2004, 31 million warfarin prescriptions were dispensed in the United States. Warfarin inhibits the activation of the vitamin K–dependent clotting factors (Factors II, VII, IX, and X) and regulatory proteins (proteins C, S, and Z). It is one of the leading drugs implicated in emergency room visits for adverse drug reactions. Annually the frequency of bleeding complications associated with overanticoagulation is 15% to 20%, with fatal bleeds measuring as high as 1% to 3%. The most effective method of warfarin reversal involves the use of Four Factor Prothrombin Complex Concentrate (PCC), which is widely used throughout Europe but is unavailable in the United States. The current therapies available to emergency room physicians in the United States are fresh frozen plasma, recombinant Factor VIIa (rFVIIa), Factor Eight Inhibitory Bypassing Activity, or Three Factor PCC concomitantly administered with vitamin K. We review the advantages and disadvantages of these therapies and recommend Three Factor PCC with small doses of rFVIIa and with vitamin K in life-threatening situations if Four Factor PCC is unavailable.
INTRODUCTION
In 2002 it was estimated that more than 3 million people, or 1.6% of the US population, were taking an oral vitamin K antagonist (VKA) such as warfarin. In men and women who were 65 years old or older the percentages were as high as 8% and 4%, respectively.1 VKAs are routinely used for the primary and secondary prevention of arterial and venous thromboembolism in patients with prosthetic heart valves, atrial fibrillation, peripheral arterial disease, antiphospholipid syndrome, and recurrent myocardial or cerebral infarction.2–4 Warfarin is the most commonly prescribed VKA worldwide and belongs to a group of drugs known as coumarins. It works by inhibiting the C1 subunit of the enzyme vitamin K epoxide reductase (VKOR), which is necessary for the activation of the vitamin K–dependent coagulation factors (Factors II, VII, IX, and X [Figure 1]) and regulatory proteins (proteins C, S, and Z).5
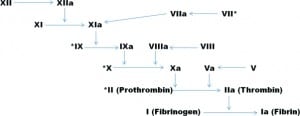
The challenge with coumarin therapy is balancing the benefit of anticoagulation versus the inherent risk of bleeding due to the functional deficiency of the coagulation factors. The frequency of warfarin-induced bleeding is 15% to 20% per year, with life-threatening or fatal bleeding rates as high as 1% to 3% per year.6–9 As the US population ages, the number of patients taking warfarin, as well as the number of patients presenting to the emergency room as a result of complications, will likely increase. Emergency room physicians should be aware of current and future therapies for rapid reversal of anticoagulant therapy.
VITAMIN K ANTAGONIST
Warfarin therapy can be problematic as a result of its narrow therapeutic index, highly variable dosage requirements among patients, and significant drug, dietary, and disease interactions.5Bleeding can result from gross abnormalities, such as hemophilia A or B, or from more subtle single nucleotide polymorphic mutations, such as those found in cytochrome P450 2C9 (CYP2C9), in vitamin K epoxide reductase, or in Factor IX propetide. These often-undiagnosed, subtle changes can increase the likelihood of a hemorrhagic complication associated with warfarin therapy.
CYP2C9 is a microsomal enzyme partially responsible for coumarin breakdown. Mutated CYP2C9 results in reduced coumarin metabolism and, subsequently, a longer half-life, thereby increasing the risk of hemorrhage with a standard dose of warfarin.5 VKOR is responsible for reducing vitamin K to its active form, which is necessary for the activation of the vitamin K–dependent clotting factors. Mutated VKOR leads to insufficient levels of reduced vitamin K and, consequently, vitamin K–dependent clotting factors.5 The addition of VKA therapy would compound this deficiency and increase the patient’s risk of hemorrhaging. In addition, a point mutation in the Factor IX propeptide results in extremely low levels of Factor IX during warfarin treatment, which in turn leads to an increased risk of bleeding.5,10
In addition to pharmacogenetic considerations there are also important drug, dietary, and disease interactions to be aware of with coumarin therapy. While it is outside the scope of this review to list every drug or supplement that may interact with coumarins, Ansell et al5 provide a comprehensive list in their 2008 article. As a result of their routine use in emergency departments, it is important to note that aspirin and a variety of antibiotics (among them levofloxacin, azithromycin, trimethoprim/sulfamethoxazole, and second- and third-generation cephalosporins) augment or potentiate the coumarin response, while barbiturates, rifampin, and cholestyramine increase clearance or reduce absorption.5,11 Diet can also play a key role in coumarin therapy—too much vitamin K intake can reduce the response to VKA therapy, while fat malabsorption or reduced vitamin K intake can potentiate the coumarin response. Hepatic dysfunction and hypermetabolic states, such as fever, also potentiate or increase the responsiveness to VKA therapy.5
Assessment of anticoagulation due to VKA therapy is typically done using the international normalized ratio (INR), which is the ratio of a patient’s prothrombin time compared to a standard prothrombin time. The prothrombin time measures 3 of the 4 vitamin K–dependent coagulation factors (Factors II, VII, and X).5 A normal INR is between 0.8 and 1.2. The INR for patients on VKA therapy varies according to the underlying condition but is typically between 2.0 and 3.5.2,4 An INR of less than 2.0 is associated with increased risk of thromboembolic events, while an INR of greater than 4.0 is associated with increased risk of bleeding.12
VKA therapy can be difficult to control and needs to be closely tracked in order to ensure patient safety. In a 2002 study,12 the medical records of 1,020 patients (12,897 INR values) receiving warfarin were retrospectively analyzed to determine how much time was spent either above or below the target INR. The authors reported a total of 3,810 (29.5%) INR values that were either above 4.0 or below 2.0; of these, 3,484 (27%) were considered to be outside of the target range. A separate study13 looked at INR values among patients with atrial fibrillation and found that only 43.7% of the INRs were within the target range. A study by Van Leeuwen et al14 found that patients who spent more time outside of their target INR range had a 2.6-fold increased risk of bleeding.
ADVERSE EFFECTS OF VKAs
In 1 year, up to 6.5% of patients on anticoagulant therapy will experience a major bleeding event affecting their soft tissue, gastrointestinal tract, or urinary tract. Approximately 1% of patients will develop a fatal bleed, often an intracranial hemorrhage (ICH).8,15,16 It was estimated in 2007 that 8,000 to 10,000 cases of coumarin-associated ICH occurred in the United States.17 In a cohort study18 of 11,526 patients with nonvalvular atrial fibrillation it was reported that those patients receiving VKA therapy were 1.97 times more likely to suffer from ICH as those not on coumarin therapy.
A prospective, multicenter study21 involving 2,745 first-time patients was conducted to assess bleeding complications associated with VKA therapy. A total of 153 bleeding events were reported (5 fatal cerebral hemorrhages, 23 major hemorrhages, and 125 minor hemorrhages) during the course of the study. The authors found 3 variables leading to an increased risk of hemorrhage: age (greater than 70 years), the first 90 days of treatment, and intensity of anticoagulation (INR greater than 4.5). Patients over 70 years of age or those in the first 90 days of treatment were 1.75 times as likely to suffer a bleeding complication as those under age 70 or those outside of the 90-day window. Those patients with an INR of 4.5 or greater were 7.91 times as likely experience to bleeding as those with an INR under 4.5.19 Other studies20–23 have reported history of bleeding, chronic alcohol abuse, disease, and concurrent use of other drugs as additional factors that increase the risk of hemorrhage.
TREATMENT OPTIONS FOR REVERSAL OF OVERANTICOAGULATION
There are several methods with which to reverse the anticoagulant effect of warfarin, including the omission of a dose of warfarin, administration of an oral or intravenous dose of vitamin K, use of fresh frozen plasma (FFP), Three- or Four-Factor Prothrombin Complex Concentrate (3F PCC, 4F PCC), recombinant Factor VIIa (rFVIIa), or the use of Factor Eight Inhibitor Bypassing Activity (FEIBA) (Figure 2). The clinical situation (absence of bleeding, major bleed, etc) must be evaluated in addition to the patient’s INR to determine the appropriate method to use to reverse the anticoagulation. The American College of Chest Physicians has released a set of guidelines for the reversal of anticoagulation therapy (Table).5

The simplest method of reversal is the withholding of 1 or more doses of the VKA. Although the coagulopathy begins to correct within 24 to 36 hours, it will not fully correct for 3 to 5 days; therefore, this method is only appropriate for an asymptomatic patient with an elevated INR or a very minor bleed.24,25 For a patient who is at a high risk of bleeding, oral vitamin K must be administered in concert with the omission of a dose of warfarin.5,26
Since the mechanism of coumarin action is competitive inhibition of VKOR with a subsequent decrease in the activity of the vitamin K–dependent coagulation factors, vitamin K itself can be used as a treatment. Administration of vitamin K overpowers the anticoagulation system and turns on the endogenous activation of the coagulation factors. Vitamin K can be administered subcutaneously, orally, or intravenously. Subcutaneous vitamin K is not significantly different from placebo in terms of correcting aberrant INRs.27 Although both oral and intravenously administered vitamin K are effective at correcting supratherapeutic INRs at 24 hours posttreatment, intravenous vitamin K can correct the INR much sooner, in as few as 4 to 6 hours.5,28 While intravenous vitamin K has repeatedly been proven effective, it is not without risks; it has been associated with severe anaphylactic reactions. For this reason, the American College of Chest Physicians, the American Heart Association, the American College of Cardiology, the Australasian Society of Thrombosis and Haemostasis, and the American Society of Hematology have suggested that the use of intravenous vitamin K be limited to life-threatening situations.3,5,29,30
There are 2 other interventions available to be used in patients with a major hemorrhage (Figure 2). FFP was the most commonly used agent for the replacement of coagulation factors in the United States as of 2008.29 It contains vitamin K–dependent coagulation Factors II, VII, and X; however, it lacks sufficient levels of factor IX. As of 2008 the American College of Chest Physicians, the Australasian Society of Thrombosis and Haemostasis, and the British Committee for Standards in Haematology have all released guidelines for the use of FFP with supratherapeutic INR. While these recommendations are similar, there are noticeable differences. The Australasian guidelines recommend considering the use of 150 to 300 mL of FFP with an INR of greater than 9.0 even without bleeding. In contrast, both the British and American guidelines do not mention the use of FFP until major bleeding is present. With major bleeding, all 3 guidelines recommend FFP use at 15 cc/kg only when PCCs are not available. In addition, the American guidelines specifically recommend PCC over FFP in cases of life-threatening bleeding.31
There are a significant number of concerns about the use of FFP in coumarin-associated overanticoagulation. As a blood product prepared from either whole blood or plasmapheresis, FFP must be ABO matched to the patient before transfusion can begin. Another issue contributing to the speed with which FFP can be administered is the fact that it requires time to thaw (since it is stored at 4°C) before it can be given to the patient.32 A retrospective study by Lee et al33 found that a median time of 6.5 hours was needed to infuse 5 U of FFP to patients who suffered from warfarin-induced ICH. As with all blood products, FFP carries a risk, however slight, of pathogen transmission; it is recommended that patients receiving large or repeated doses of FFP be vaccinated for both hepatitis A and B.32 Of particular concern is the varying levels of coagulation factors seen in FFP, which may result in a partial or insufficient reversal of INR.28 Perhaps most worrying for the use of FFP in overanticoagulated patients is the sheer volume that is often required to reverse the coagulation defect: it is not uncommon to rapidly transfuse 2 to 4 L (approximately 8–16 U) in patients with greatly elevated INRs.17 Rapid transfusion of large volumes can lead to cardiogenic lung edema, and transfusion of FFP specifically has been associated with a noncardiogenic lung edema known as transfusion-related acute lung injury (TRALI).34,35 While immunogenic TRALI is rare (1 per 5,000 transfusions), it requires mechanical ventilation in 70% of cases and is fatal in 6% to 9% of cases.34Because of these limitations, FFP is recommended for use only in situations where other, more effective therapeutics are not readily available.4,28,30,36,37
rFVIIa and FEIBA are newer therapies for warfarin reversal in bleeding patients. rFVIIa activates Factor X to Factor Xa, allowing the formation of thrombi.38 Several small studies39–43 have shown that rFVIIa can quickly correct supratherapeutic INRs with doses ranging from 10 to 90 μg/kg. However, the majority of the patients in these studies also received FFP and vitamin K; therefore, it is unclear if rFVIIa alone can be effective in acute warfarin reversal. Additionally, its high cost for an average patient ($5,542 for an average dose versus $2,205 for the same patient on PCC) and short half-life of 2.5 to 3 hours make it a less favorable candidate with which to treat warfarin-induced coagulopathy.44 The incidence of thrombotic adverse events associated with rFVIIa is rare, 24.5 per 105 infusions, with an elevated risk for cerebrovascular thrombosis.45
FEIBA is an activated PCC. It contains activated Factor VII and small amounts of activated Factors II, IX, and X.46 A study by Wójcik et al47 found that 56 of 72 nonhemophiliac patients (77.8%) with life-threatening bleeds associated with warfarin survived after treatment with FEIBA concomitantly administered with 10 mg of vitamin K. The INR of patients receiving FEIBA normalized 12 times faster than did that of those receiving FFP. Like rFVIIa, FEIBA contains the activated form of Factor VII, but it is not as costly.47 A pharmacovigilance study45 found the incidence of thrombotic adverse events associated with FEIBA to be 8.24 per 105 infusions. The overwhelming majority of research on FEIBA has been conducted with hemophiliac patients; therefore, more studies need to be done with patients on warfarin therapy.
While 4F PCC contains all the vitamin K–dependent coagulation factors and is widely used in European countries, it has not been approved by the Food and Drug Administration for use in the United States. 3F PCC, which lacks adequate levels of Factor VII in relation to Factors II, IX and X, is in limited use in the United States. A recent study48 showed that 3F PCC administered alone cannot adequately lower the supratherapeutic INR levels to less than 3 in 50% of patient as a result of its low levels of Factor VII. Only after the administration of FFP in concert with 3F PCC were INR levels sufficiently corrected.
The most effective product available for the rapid reversal of overanticoagulation is 4F PCC.16 3F PCC and 4F PCC have a number of advantages over FFP. While FFP must be thawed before use, PCCs are stored as a lyophilized powder at room temperature and are reconstituted in sterile water immediately prior to use, which means they can be administered much more quickly than FFP.28Additionally, PCCs typically undergo 2 viral inactivation steps and are further purified and pooled, making them safer than FFP in terms of the transmission of pathogens, especially HIV and hepatitis B and C. This purification process also obviates the need for ABO matching.4,28,29,49,50 Finally, PCCs offer known quantities of the vitamin K–dependent coagulation factors in small volumes, which substantially reduces the risks associated with large-volume infusions of FFP. The concentration of the coagulation factors in FFP is only 4% of what is present in 4F PCC, making it necessary to administer higher volumes of FFP (2–4 L of FFP vs 20–50 U/kg of PCC) to have the same effect.51 In a retrospective study37 of 17 patients with anticoagulant-related ICH treated with either FFP or 4F PCC, it was discovered that INRs of patients treated with 4F PCC were reversed 4 to 5 times more rapidly than those of patients treated with FFP. Similarly, in a prospective study52 of 41 patients requiring rapid reversal of VKA-associated overanticoagulation, 28 of 29 patients treated with 4F PCC showed a complete correction of the INR within 15 minutes (mean INR of 1.3), while 0 of 12 patients treated with FFP corrected within this time frame (mean INR of 2.3). A number of other studies15,24,49,50,53–58 have confirmed the efficacy of PCCs in the rapid reversal of elevated INRs.
The main concerns associated with PCCs are the risks of thromboembolism. In a meta-analysis of 14 studies with 460 patients, only 7 thromboembolic complications were observed. All of these complications occurred in only 3 of the 14 studies. Additionally, 4 of the 7 thrombotic events were “likely linked to underlying patient characteristics” rather than to administration of PCC.59 Some studies have indicated a low incidence of thromboembolic events following administration of PCCs, but the incidence of such events has appeared to decrease in recent years. This may be due to newer formulations, which include the inhibitors of coagulation, such as proteins C, S, and Z, or to an improved balance of coagulation factors in the concentrate.60 It appears that the risk of thrombosis is related both to the dose of PCC administered and to underlying patient characteristics.54 Several recent studies49,50,55,57 have shown a transient elevation in markers of coagulation activation but no clinical evidence of thromboembolism with modern PCC formulations.
ADMINISTRATION OF 3F AND 4F PCC
PCC was originally developed to treat hemophilia B (Factor IX deficiency). Therefore, dosing of PCC is based on the treatment of this disease: 25 to 50 IU of Factor IX/kg of body weight.29,52,61 The “standard” dose of 20 mL/kg, or 500 IU, of PCC is commonly used in the treatment of overanticoagulation.54,62 Despite this, newer research indicates that individualized dosing, based on the body weight of the patient, their initial INR, and their target INR, may be more effective than the standard dose at reversing the effect of warfarin. An open, prospective randomized controlled trial54looked at 93 patients who needed acute reversal of their anticoagulation therapy (47 patients treated with the standardized dose of PCC and 46 patients treated with an “individualized” dose) and found the individualized group had 89% of patients reach their target INR 15 minutes posttreatment, compared with only 43% of patients treated with the standard dose (P < 0.001). Numerous other studies28,54,61,63 have used “semi-individualized” dosing of PCC (25, 30/35, or 50 IU/kg) based on the patient’s INR at the time of presentation. PCC, along with FFP, must be administered with 5 to 10 mg intravenous vitamin K.16,26,52,54,55
CONCLUSION
Currently there exists no optimal therapy for anticoagulant overdose. Emergency room physicians are caught balancing the cost of rFVIIa with the therapeutic delay of FFP and suboptimal reversal of 3F PCC. 4F PCC, which appears to better meet the needs of overanticoagulated and bleeding patients, is available in Europe but not in the United States. Therefore, the authors recommend the use of 3F PCC with vitamin K and a judicious amount of rFVIIa or FEIBA to balance timeliness with cost, especially in patients with critical bleeds, until such time that 4F PCC becomes available in the United States.
Footnotes
Supervising Section Editor: Eric R. Snoey, MD
Submission history: Submitted July 29, 2010; Revision received September 22, 2010; Accepted March 21, 2011
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.3.2051
Address for Correspondence: Sean Henderson, MD
Department of Emergency Medicine, LACþUSC Medical Center, 1200 N State St, Rm 1011, Los Angeles, CA 90033
E-mail: sohender@hsc.usc.edu.
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone Survey. J Am Med Assoc. 2002;;287:337–344.
2. Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition–2005 update. Br Soc Haematol. 2005;;132:277–285.
3. Hirsch J, Fuster V, Ansell J, et al. American Heart Association/American College of Cardiology Foundation Guide to Warfarin Therapy. J Am Coll Cardiol. 2003;;41:1633–1652. [PubMed]
4. Schulman S. Care of patients receiving long-term anticoagulant therapy. N Engl J Med.2003;;349:675–683. [PubMed]
5. Ansell J, Hirsch J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed.) Chest.2008;;133:160–198.
6. Fihn SD, Callahan CM, Martin DC, et al. The risk for and severity of bleeding complications in elderly patients treated with warfarin. Ann Intern Med. 1996;;124:970–979. [PubMed]
7. Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT) Lancet. 1996;;348:423–428. [PubMed]
8. Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;;105:91–99. [PubMed]
9. Hylek EM, Regan S, Go AS, et al. Clinical predictors of prolonged delay in return of the international normalized ratio to within the therapeutic range after excessive anticoagulation with warfarin. Ann Intern Med. 2001;;135:393–400. [PubMed]
10. Chu K, Wu SM, Stanley T, et al. A mutation in the propetide of Factor IX leads to warfarin sensitivity by a novel mechanism. J Clin Invest. 1996;;98:1619–1625. [PMC free article] [PubMed]
11. Glasheen JJ, Fugit RV, Prochazka AV. The risk of overanticoagulation with antibiotic use in outpatients on stable warfarin regimens. J Gen Intern Med. 2005;;20:653–656. [PMC free article][PubMed]
12. Wittkowsky AK, Devine EB. Frequency and causes of overanticoagulation and underanticoagulation in patients treated with warfarin. Pharmacotherapy. 2004;;24:1311–1316.[PubMed]
13. Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation. Arch Intern Med. 2000;;160:967–973. [PubMed]
14. Van Leeuwen Y, Rosendaal FR, Cannegieter SC. Prediction of hemorrhagic and thrombotic events in patients with mechanical heart valve prostheses treated with oral anticoagulants. J Thromb Haemost. 2008;;6:451–456. [PubMed]
15. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;;95:315–328. [PubMed]
16. Hanley JP. Warfarin reversal. J Clin Pathol. 2004;;57:1132–1139. [PMC free article] [PubMed]
17. Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;;82:82–92. [PubMed]
18. Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation. J Am Med Assoc. 2003;;290:2685–2692.
19. Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT) Lancet. 1996;;348:423–428. [PubMed]
20. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed.) Chest. 2008;;133:257–298.
21. Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;;115:2689–2696.[PubMed]
22. Hylek EM, Chang Y, Skates SJ, et al. Prospective study of the outcomes of ambulatory patients with excessive warfarin anticoagulation. Arch Intern Med. 2000;;160:1612–1617. [PubMed]
23. McMahan DA, Smith DM, Carey MA, et al. Risk of major hemorrhage for outpatients treated with warfarin. J Gen Intern Med. 1998;;13:311–316. [PMC free article] [PubMed]
24. Makris M. Optimisation of the Prothrombin Complex Concentrate dose for warfarin reversal.Thromb Reversal. 2005;;115:451–453.
25. White RH, McKittrick T, Hutchinson R, et al. Temporary discontinuation of warfarin therapy: changes in the international normalized ratio. Ann Intern Med. 1995;;122:40–42. [PubMed]
26. Makris M, van Veen J, Maclean R. Warfarin anticoagulation reversal: management of the asymptomatic and bleeding patient. J Thromb Thrombolysis. 2010;;29:171–181. [PubMed]
27. DeZee KJ, Shimeall WT, Douglas KM, et al. Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis. Arch Intern Med. 2006;;166:391–397. [PubMed]
28. Makris M, Watson HG. The management of coumarin-induced over-anticoagulation. Br J Haematol. 2001;;114:271–280. [PubMed]
29. Baker RI, Coughlin PB, Gallus AS, et al. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust. 2004;;181:492–497. [PubMed]
30. Dentali F, Crowther MA. Management of excessive anticoagulant effect due to vitamin K antagonists. Hematology. 2008. pp. 266–270. [PubMed]
31. Levine M, Goldstein JN. Emergency warfarin reversal. In: Edardes JP, editor. Coumarin Anticoagulant Research Progress. New York, NY: Nova Science Publishers Inc;; 2008. pp. 1–9.
32. Duguid J, O’Shaughnessy DF, Atterbury C, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br Soc Haematol. 2004;;126:11–28.
33. Lee SB, Manno EM, Layton KF, et al. Progression of warfarin-associated intracerebral hemorrhage after INR normalization with FFP. Neurology. 2006;;67:1272–1274. [PubMed]
34. Bux J. Transfusion-related acute lung injury (TRALI): a serious adverse event of blood transfusion. Vox Sanguinis. 2005;;89:1–10. [PubMed]
35. Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;;131:1308–1314.[PubMed]
36. Dentali F, Ageno W, Crowther M. Treatment of coumarin-associated coagulopathy: a systematic review and proposed treatment algorithms. J Thromb Haemost. 2006;;4:1853–1863. [PubMed]
37. Fredriksson K, Norrving B, Stromblad LG. Emergency reversal of anticoagulation after intracerebral hemorrhage. Stroke. 1992;;23:972–977. [PubMed]
38. Rowe S, Turner RM. Coagulation Factor VIIa (recombinant) for warfarin-induced intracranial hemorrhage. Am J Health-Syst Pharm. 2010;;67:361–365. [PubMed]
39. Rosovsky RP, Crowther MA. What is the evidence for the off-label use of recombinant Factor VIIa (rFVIIa) in the acute reversal of warfarin? Hematology. 2008. pp. 36–38. [PubMed]
40. Sørensen B, Johansen P, Nielsen GL, et al. Reversal of the international normalized ratio with recombinant activated Factor VII in central nervous system bleeding during warfarin thromboprophylaxis: clinical and biochemical aspects. Blood Coagulation Fibrinolysis.2003;;14:469–477. [PubMed]
41. Freeman WD, Brott TG, Barrett KM, et al. Recombinant Factor VIIa for rapid reversal of warfarin anticoagulation in acute intracranial hemorrhage. Mayo Clin Proc. 2004;;79:1495–1500. [PubMed]
42. Brody DL, Aiyagari V, Shackleford AM, et al. Use of recombinant Factor VIIa in patients with warfarin-associated intracranial hemorrhage. Neurocrit Care. 2005;;2:263–267. [PMC free article][PubMed]
43. Dager WE, King JH, Regalia RC, et al. Reversal of elevated international normalized ratios and bleeding with low-dose recombinant activated Factor VII in patients receiving warfarin.Pharmacology. 2006;;26:1091–1098.
44. Safaoui M, Aazami R, Hotz H, et al. A promising new alternative for the rapid reversal of warfarin coagulopathy in traumatic intracranial hemorrhage. Am J Surg. 2009;;197:785–790. [PubMed]
45. Aledort LM. Comparative thrombotic event incidence after infusion of recombinant Factor VIIa versus Factor VIII Inhibitor Bypassing Activity. J Thromb Haemost. 2004;;2:1700–1708.[PubMed]
46. Tjønnfjord GE, Holme PA. Factor eight inhibitor bypass activity (FEIBA) in the management of bleeds in hemophilia patients with high-titer inhibitors. Vasc Health Risk Manage. 2007;;3:527–531.
47. Wójcik C, Schymik ML, Cure EG. Activated prothrombin complex concentrate Factor VIII Inhibitor Bypassing Activity (FEIBA) for the reversal of warfarin-induced coagulopathy. Int J Emerg Med. 2009;;2:217–225. [PMC free article] [PubMed]
48. Holland L, Warkentin TE, Refaai M, et al. Suboptimal effect of a Three-Factor Prothrombin Complex Concentrate (Profilnin-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion. 2009;;49:1171–1177. [PubMed]
49. Lubetsky A, Hoffman R, Zimlichman R, et al. Efficacy and safety of a Prothrombin Complex Concentrate (Octaplex) for rapid reversal of oral anticoagulation. Thromb Res. 2004;;113:371–378.[PubMed]
50. Ostermann H, Haetrel S, Knaub S, et al. Pharmacokinetics of Beriplex P/N Prothrombin Complex Concentrate in healthy volunteers. Thromb Haemost. 2007;;98:790–797. [PubMed]
51. Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfusion Med Rev.2007;;21:37–48.
52. Makris M, Greaves M, Phillips WS, et al. Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;;77:477–480. [PubMed]
53. Nitu IC, Perry DJ, Lee CA. Clinical experience with the use of clotting factor concentrates in oral anticoagulation reversal. Clin Lab Haematol. 1998;;20:363–367. [PubMed]
54. van Aart L, Eijkhout HW, Kamphuis JS, et al. Individualized dosing regimen for Prothrombin Complex Concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: an open, prospective randomized controlled trial. Thromb Res. 2006;;118:313–320.[PubMed]
55. Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced overanticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;;115:998–1001. [PubMed]
56. Lorenz R, Kienast J, Otto U, et al. Efficacy and safety of a Prothrombin Complex Concentrate with two virus-inactivation steps in patients with severe liver damage. Eur J Gastroenterol Hepatol.2003;;15:15–20. [PubMed]
57. Riess HB, Meier-Hellmann A, Motsch J, et al. Prothrombin Complex Concentrate (Octaplex) in patients requiring immediate reversal of oral anticoagulation. Thromb Res. 2007;;121:9–16.[PubMed]
58. Cartmill M, Dolan G, Byrne JL, et al. Prothrombin Complex Concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg. 2000;;14:458–461. [PubMed]
59. Leissinger CA, Blatt PM, Hoots WK, et al. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;;83:137–143. [PubMed]
60. Levy JH, Tanaka KA, Dietrich W. Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology. 2008;;109:918–926. [PubMed]
61. Preston FE, Laidlaw ST, Sampson B, et al. Rapid reversal of oral anticoagulation with warfarin by a Prothrombin Complex Concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol.2002;;116:619–624. [PubMed]
62. Yasaka M, Sakata T, Naritomi H, et al. Optimal dose of Prothrombin Complex Concentrate for acute reversal of oral anticoagulation. Thromb Res. 2005;;115:455–459. [PubMed]
63. Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the use of antithrombin concentrates and Prothrombin Complex Concentrates. Blood Transfusion. 2009;;7:325–334.[PMC free article] [PubMed]


