| Author | Affiliation |
|---|---|
| Chad S Kessler, MD | Jesse Brown VA Medical Center, Department of Emergency Medicine, Chicago, IL University of Illinois at Chicago, Chicago, IL |
| Julie Bauml | University of Illinois at Chicago, Chicago, IL |
ABSTRACT
Although true urologic emergencies are extremely rare, they are a vital part of any emergency physician’s (EP) knowledge base, as delays in treatment lead to permanent damage. The four urologic emergencies discussed are priapism, paraphimosis, testicular torsion, and Fournier’s gangrene. An overview is given for each, including causes, pathophysiology, diagnosis, treatment, and new developments. The focus for priapism is on diagnosis and distinguishing high-flow from low-flow forms, as the latter requires emergent treatment. For paraphimosis, we describe various methods of relieving the stricture, from manual reduction to surgery in extreme cases. For testicular torsion, the most important factor in salvaging the testicle is decreasing time to treatment. This is accomplished through experience and understanding which signs and symptoms strongly suggest it, so that time-consuming tests are avoided. Lastly, Fournier’s gangrene is potentially fatal. While aggressive medical and surgical therapy will improve chances of survival and outcome, it is vital for the emergency department (ED) physician to diagnose Fournier’s. It often presents in the elderly, immunocompromised, or those with depressed mental status. The goal of this paper is to arm EPs with information to recognize urological emergencies and intervene quickly to preserve tissue, fertility, and life.
PRIAPISM
Overview
Priapism is a persistent and painful erection lasting more than four hours that is not related to sexual arousal or relieved by sexual intercourse.1 There are two types of priapism: low-flow and high-flow. Low-flow priapism, or ischemic priapism, results from decreased venous and lymphatic drainage of the corpus cavernosum. Highflow priapism is less likely to be ischemic and is most often caused by a traumatic arterial laceration. The main complication of priapism is erectile dysfunction, especially in recurrent ischemic priapism, due to inflammation and fibrosis of the corpus cavernosum.
Causes
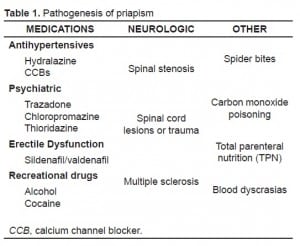
Most cases of low-flow priapism in adults are secondary to medication or drug use. Drugs of abuse, such as alcohol and cocaine, are important contributors to priapism, especially in heavy users.2Blood pressure medications such as hydralazine, prazosin, and calcium channel blockers are common causes. Psychiatric medications, also notorious for causing priapism, include trazodone, chlorpromazine, thioridazine, and other selective serotonin re-uptake inhibitors (SSRIs). Oral erectile dysfunction medications like sildenafil, vardenafil, and tadalafil can also lead to priapism (Table).3 Furthermore, blood dyscrasias and hypercoagulable states, such as sickle cell disease, thalassemias, polycythemia, and vasculitis, often cause low-flow priapism.1 The most common cause of high-flow priapism, regardless of age, is arteriovenous fistula formation secondary to perineal trauma, most commonly straddle-type injuries.4
Pathophysiology
Several underlying mechanisms cause priapism. In low-flow priapism, the most common mechanism is sludging of red blood cells, which clogs the spaces of the corpus cavernosum leading to impaired drainage of blood.5 Impaired venous outflow can also lead to thrombosis and further ischemia from remaining stagnant blood.3 Highflow priapism is usually caused by an arteriovenous shunt following perineal trauma, which causes abnormally high flow of blood through the caverosum.5
Diagnosis
Patients present with an erect, tender penis and soft glans, unrelated to sexual arousal.5 When comparing low- and high-flow states, it should be noted that the high-flow state is usually less painful and carries a smaller risk of ischemia.3 Since the recognition of priapism is fairly straightforward, the diagnostic challenge lies in differentiating high- from low-flow states through patient history, as the treatment differs greatly. Generally, high-flow priapism patients will have a recent history of perineal trauma, often times a straddle injury. These patients usually seek treatment later in the course as they do not typically experience intense pain, as opposed to low-flow patients.1 The definitive distinction between high- and low-flow states can also be made by penile blood gas, aspirated from the corpus cavernosum. Low-flow priapism is a hypoxic state, so blood appears dark and dusky. Non-ischemic priapism aspirate is well oxygenated and bright red. An ischemic penile blood gas typically reveals a partial pressure of oxygen (PO2) < 30 mmHg, a partial pressure of carbon dioxide (PCO2) > 60 mmHg, and a pH < 7.25, whereas a non-ischemic state will closely mimic normal arterial blood. Color duplex ultrasonography may also be used to differentiate the two by visualizing flow patterns. Additionally, a fistula or pseudoaneurysm may be discovered by ultrasound in the high-flow state.2 Complete blood count (CBC), coagulation studies, and a sickle cell screen should be performed.1 Hemoglobin electrophoresis and reticulocyte count can reveal sickle-cell abnormalities or thalassemias and are indicated for all priapism patients unless the cause of priapism is definitively known. Moreover, a urine toxicology screen for illicit drugs is useful when suspected.2
Treatment
The first step in the management of priapism, regardless of the cause, is always hydration and analgesia. If the patient has an acute sickle-cell crisis, fluids and analgesia with supplemental oxygen may lead to detumescence.1 For ischemic priapism, subcutaneous terbutaline sulfate (0.25 to 0.5 mg) every 30 minutes as needed is often effective.3 The next step in treatment involves aspiration of cavernosal blood and direct caversonal injection of phenylephrine (100–200 mg every 5–10 minutes with a max of 1000 mg).2 A three-way stopcock is recommended so that irrigation can be alternated with aspiration.3 Blood pressure monitoring is vital if attempting to treat with phenylephrine, and often these patients need continuous cardiac telemetry. If there is immediate recurrence after aspiration, the next step is a surgical shunt between the corpora spongiosa and cavernosum.
For high-flow priapism, the treatment strategy is much different. This type of priapism is non-ischemic as arterial blood flow is intact. Thus, it is usually non-emergent and can be observed before deciding upon invasive treatment. Preserving penile function is of primary concern. Treatment with selective arterial embolization using an autologous clot is highly effective with normal sexual function restoration in 86% of cases.4
Evidence-Based Updates
Previously, priapism caused by sickle-cell anemia was treated by transfusing packed red blood cells (RBCs).5 However, that therapy has not been shown to change short- or long-term outcomes and may even cause neurological sequelae.6 Although it is important to screen for sickle cell disease as a cause of priapism, its treatment is currently not different from that of other causes. Sickle-cell priapism should be treated in the same manner as other ischemic priapisms with supplemental oxygen, analgesia and hydration. Eventual exchange transfusions can be performed, but direct blood transfusions are now controversial.3,6
New research focuses on treatment options targeting the underlying pathology of priapism. Phosphodiesterase 5 (PDE5), a molecular effector, has been implicated in recurrent priapism. PDE5 is important in breaking down nitric oxide and restoring normal blood flow to the penis after an erection. In addition, drugs which limit the fibrotic response in priapism, and therefore limit potential erectile dysfunction, are being studied. Notably, antioxidants are being investigated as a possible treatment, although effectiveness has not been established.2,7,8 The theory is that antioxidants will relieve some of the post-ischemic reperfusion injury, thus limiting cavernosal fibrosis and muscle damage.
PARAPHIMOSIS
Overview
Paraphimosis occurs when the foreskin becomes fixed in the retracted position and cannot be reduced, therefore constricting venous return from the glans. Without immediate treatment this causes edema, induration, ischemia, and eventually necrosis of the glans.
Causes
The most common cause of paraphimosis is previous phimosis (fibrosis and constriction of the prepuce distal to the glans preventing retraction).9 This leads to a circular scar, which can then form a tourniquet when the foreskin is retracted, preventing proper venous and lymphatic drainage. Another significant cause of paraphimosis is iatrogenic, when medical staff fails to reduce the foreskin after urethral catheterization or genital exam. Other causes of paraphimosis include poor urogenital hygiene, chronic balanoposthisis, and genital piercing.5
Diagnosis
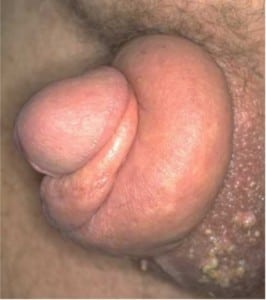
Typically, patients present with edema and pain of the glans and the inability to pull back the retracted foreskin. Diagnosis is usually straightforward as the stricture caused by the prepuce of the non-retractable foreskin and the resulting edema is easily visualized (Figure 1). Allergic reactions, trauma, and hair tourniquets can all have similar presentations to paraphimosis. When considering paraphimosis, it is important to distinguish between various infections and strictures of the penis. To clarify, balanitis is an infection of the glans only, whereas balanoposthitis is an infection of the glans and the foreskin.
Treatment
The primary concerns with regard to treatment of paraphimosis are to relieve any significant pain and prevent further ischemia to the glans. First, manual reduction of the foreskin should be attempted. A penile nerve block or ice can often minimize patient discomfort and lead to a successful decompression. The glans should be compressed to reduce the edema during the attempt to replace the foreskin. If the foreskin cannot be reduced within a few attempts this effort should be terminated so as not to cause more irritation and swelling. Needle punctures into the inflamed portion of the penis to remove blood can help decrease the size of the glans and help facilitate manual reduction as well. If these measures fail, a urologist should be consulted to perform a dorsal slit procedure. A dorsal slit procedure entails incising the fibrotic ring of the prepuce to relieve the constriction and reduce the foreskin. Circumcision is the definitive treatment and will prevent any future episodes.3,5
Evidence-Based Updates
Alternative treatments for paraphimosis, especially popular in Europe, involve creative ways of decreasing the edema to the glans and facilitating reduction. The first concept is based on applying a high concentration of sugar to the outside of the glans in order to osmotically draw water out and reduce swelling. Granulated sugar can be directly applied, but it is not always on hand in the ED. An alternative is to soak gauze in 50mL of 50% dextrose and apply. At this time, there are no randomized controlled studies documenting the efficacy of this procedure, and evidence exists only in case reports. Another way of decreasing edema is to inject 1mL of hyaluronidase (250 U/mL) into the prepuce.11 Hyaluronidase can break down hyaluronic acid in the extracellular fluid, decreasing tissue resistance and increasing solute diffusion between tissues.12 The risk with these procedures is that they delay resolution of the ischemia to the glans by one to two hours. Therefore, although these procedures are safe, they should be attempted only after reduction with penile block alone fails and the patient wishes to avoid dorsal slit procedure or circumcision. Another method to decrease swelling that can be effective up to 90% of the time is to soak the penis in a glove full of ice for five minutes before attempting manual reduction.13 It should be noted that sufficient evidence does not exist for any of these treatments of paraphimosis. The largest case studies for these methods are as follows: use of sugar—three patients,14 iced glove—10 patients,13 and multiple needle punctures—39 patients.15 Prospective, randomized controlled studies would be invaluable in identifying the best method(s) of treating paraphimosis.
TESTICULAR TORSION
Overview
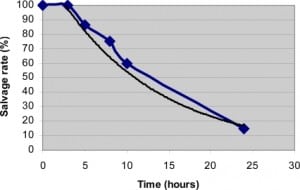
Testicular torsion results from a twisting of the spermatic cord, which impedes blood flow to the testis and impairs venous drainage, resulting in edema, ischemia, and necrosis. Testicular torsion has a bimodal age distribution with the first peak at age 1–2 years old and the second higher peak in adolescence. Torsion is fairly uncommon in adults over 40.5 Speed of diagnosis and time to treatment is vital in preserving patients’ testes and fertility. If detorsion occurs within four hours, the salvage rate is 96%, which drops precipitously to 10% after 24 hours.3 Although not always the cause of an acute scrotum, testicular torsion needs to remain high on the differential until proven otherwise as “time is testicle.” Important complications of testicular torsion include infertility and possible autoimmune attack of the contralateral testicle, as a response to the destruction of necrotic tissue and breakdown of the blood-testicular barrier in the affected testicle.16
Pathophysiology
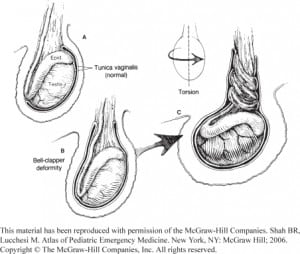
The basic pathophysiology behind testicular torsion is a malformation in which the testicle is allowed to rotate more freely around the spermatic cord. The most common abnormality is a malformed tunica vaginalis. The tunica vaginalis normally attaches the superior pole of the testes to the posterior scrotum, fixing it in place. When the vaginalis instead extends over the whole testicle, fixing it in a horizontal position, this is called a “bell-clapper deformity” (Figure 2), which predisposes to torsion.3 This deformity is present in up to 12% of the male population.18 In adults, incomplete attachment of the gubernacula is a more common predisposing malformation.5 Both malformations leave the testicle free to rotate. Furthermore, torsion might also be caused by strong cremasteric reflex during nocturnal erections, although this has yet to be well established in the literature.1
Diagnosis
The most common presentation is that of acute scrotal pain and swelling, often with a high-riding testicle and an absent cremasteric reflex. History is extremely important in differentiating testicular torsion from other common causes of acute scrotum, such as appendiceal torsion and epidydimitis.1Testicular torsion has a quicker onset of pain, whereas epidydimitis and appendiceal torsion typically have more subacute presentations.3 Patients with testicular torsion often have experienced previous bouts of pain indicating intermittent torsion. Testicular appendages are embryologic remnants of ductal structures. Torsion of these structures causes local hemorrhage, which often presents with a bluish discoloration on the skin. The presence of localized tenderness over the superior pole of the testis along with this “blue dot sign” is highly suggestive of appendiceal torsion. Early appendiceal torsion may be differentiated by history and exam from testicular torsion, but as it progresses, swelling obscures the blue dot sign and makes point tenderness of the superior pole more difficult to appreciate.18 Moreover, epidydimitis is more common after puberty (due to sexual activity) and torsion of the appendage is more common in prepubertal boys. Additionally, palpation of the spermatic cord usually reveals tenderness in epidydimitis but not in torsion.19 A positive Prehn’s sign (relief of pain upon elevation of the scrotum) points away from torsion and suggests epidydimitis but is an unreliable test with poor sensitivity.5 The cremasteric reflex is intact in epidydimitis but absent in testicular torsion. Absent cremasteric reflex is the most sensitive sign of testicular torsion with two retrospective studies20,21 showing 100% sensitivity for testicular torsion in boys older than 30 months.22 Urinalysis showing pyuria and leukocytosis suggests epidydimitis as do urinary symptoms such as dysuria, urgency, and frequency. Further symptoms such as nausea and vomiting are more common in testicular torsion than in appendiceal torsion or epidydimitis. In conjunction with scrotal pain, nausea and vomiting in children or adolescents has a 96% positive predictive value for torsion.1,23
In the past, nuclear scintigraphy had been used to distinguish between testicular torsion, epidydimitis, and torsion of the appendix. However, it has largely been replaced by color flow doppler where available, as it is quicker to use and simultaneously analyzes the anatomy of the spermatic cord and testicle.19 Color flow doppler is both sensitive and specific, 89.9% and 98.8% respectively.18 Computed tomography (CT) scans provide detailed anatomical information but carry a radiation exposure risk for the testicles and also fail to provide information about blood flow to the testes. Of note, obtaining diagnostic tests should not delay surgical exploration and repair. These tests are used only when history and physical provide equivocal findings.5 No one sign or symptom establishes the diagnosis of torsion. Ultimately, clinical judgment is always best in evaluating testicular pain and swelling.
Treatment
Time is of the essence in attempting to salvage an ischemic testicle, as salvage rates rely heavily on a speedy diagnosis (Figure 3). Treatment consists of emergent surgery to detorse the affected testicle and attach it properly to the scrotal wall. This procedure is also done on the unaffected testicle as malformations which contribute to testicular torsion are often bilateral.23 When suspicion of torsion is high, the only appropriate step is immediate surgical consultation to save as much tissue as possible. While waiting for surgical intervention, manual detorsion of the testicle by using a medial to lateral rotation or “dialing out” can be attempted. If successful, this can restore some blood flow and provide temporary pain relief. However, it is not definitive treatment and surgical consultation is still required.3,5
Evidence-Based Updates
In most instances, surgical repair is the best and most appropriate treatment for testicular torsion. However, there is new research focused on the post-surgical setting looking at medical treatments that may limit reperfusion injury and help preserve testicular function. The list of proposed medications is exhaustive with more than 40 researched agents, usually investigated in rats. The most frequently discussed medications include allopurinol, melatonin, N-acetylcysteine, zinc aspartate, and caffeic acid phenethyl ester (CAPE). Allopurinol has mixed results in rat trials with one study showing no histological improvements after reperfusion versus control25 and another showing significant improvement in tissue damage when using lipid peroxidation byproducts as a surrogate marker for oxidative injury.26 Another study illustrated biochemical changes and decreased injury with allopurinol compared with melatonin that demonstrated histological improvement as well.27 N-acetylcysteine has been shown to decrease post-reperfusion apoptosis of germ cells in rats,28 and zinc aspartate and CAPE have shown decreased histological damage.29,30All of the preceding studies are relatively new and require further animal and human testing before clinical use.
FOURNIER’S GANGRENE
Overview
Fournier’s gangrene is a necrotizing fasciitis of the perineum which can quickly spread to the skin of the entire scrotum and penis. It is usually caused by a polymicrobial infection. Fournier’s affects all ages and both genders. This condition is life-threatening with a mortality rate of 13–22% despite timely and aggressive therapy.5,31
Causes
In Fournier’s gangrene, 50–60% of infections stem from a gastrointestinal (GI) or genitourinary (GU) source. Immunocompromised patients, especially diabetics and alcoholics, with or without trauma or instrumentation are at significant risk. Additionally, the infection is most aggressive when both aerobic and anaerobic bacteria are involved.3 The most commonly cultured organisms include E. coli, bacteroides, and staphylococci. The most likely culprit for an infection of colorectal origin isclostridium.5
Pathophysiology
The process begins locally with infection in the skin and spreads down the fascial plane where inflammation, ischemia, and necrosis result. The low oxygen content and necrosis potentiate the effects of the anaerobic bacteria and cause rapid dissemination of the infection.3
Diagnosis
Patients typically present with genital induration, pain, erythema, and crepitus. A plain radiograph or CT may demonstrate air in the perineal tissues.1 Although diagnosis is straightforward when the lesions are found (Figure 4), failure to examine the genitals, especially in the elderly or obtunded patient, can result in misdiagnosis. Additionally, finding the nidus of infection, if one exists, is important. If a periurethral source is suspected, a retrograde urethrogram should be performed. If a perirectal source is suspected, proctoscopy may be revealing.5 It is also important to elicit a history of perineal trauma, as even superficial scratches or burns can be the initiating event.
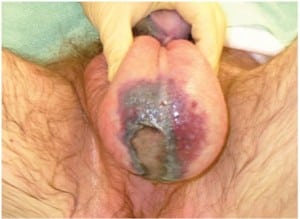
Treatment
Treatment of Fournier’s gangrene relies on an aggressive medical and surgical approach. Medical treatment includes rapid fluid resuscitation and broad spectrum antibiotics to cover gram positive, gram negative, clostridium and anerobes.3 Surgical debridement is the most important treatment and should be followed by aggressive wound care with frequent dressing changes and re-debridement if required.31 Genital skin is highly elastic and grafts are not required unless over 60% of the skin is removed. A cosmetically acceptable scrotum can generally be restored post-debridement. Suprapubic catherterization may be necessary depending on the extent of the damage.1
Evidence-Based Updates
Hyperbaric oxygen (HBO) has traditionally been used as adjunctive therapy for Fournier’s, although randomized controlled trials of effectiveness are lacking. Studies preformed as recently as 2005 have failed to show a significant difference in morbidity or mortality associated with hyperbaric oxygen. Moreover, in one study, the author showed that patients required an average of six treatments at $600–$1300 each.33 A small trial in 2008 from a regional burn center with expertise in HBO echoed these findings and was also not able to show improved outcome. The generalized use of HBO for Fournier’s gangrene cannot be routinely recommended.
CONCLUSION
True urologic emergencies presenting to the ED are rare. However, the difficulty in differentiating these emergencies from more common conditions and the need for quick, decisive action, require that EPs be vigilant and knowledgeable. For priapism the major difficulty lies in differentiating high-flow from low-flow states as low-flow requires more immediate treatment. For paraphimosis, it is important to understand the various methods of reduction: manual with analgesia, dorsal slit procedure, and circumcision. Testicular torsion diagnosis relies heavily on history and physical. With high clinical suspicion, an EP should never wait for a confirmatory test to consult with an urologist. Lastly, in Fournier’s gangrene, a thorough physical exam, especially in the elderly and immunocompromised, can lead to diagnosis and prompt treatment of this potentially fatal infection. In order to decrease permanent tissue damage, reduce infertility and save lives, a vigilant EP will always be mindful of these four non-traumatic urologic emergencies.
Footnotes
The authors would like to thank Dr. Henry Pitzele and Dr. Wesley Eilbert for their time and help in reviewing this manuscript.
Supervising Section Editor: Chris Mills, MD, MPH
Submission history: Submitted January 20, 2009; Revision Received April 14, 2009; Accepted April 26, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Chad Kessler, MD, FACEP, Department of Emergency Medicine, Jesse Brown VA Hospital, 820 S. Damen Ave., Chicago, IL 60612
Email Chad.kessler@va.gov
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Rosenstein D, McAninch J. Urologic Emergencies. Med Clin North Am. 2004;88:495–518.[PubMed]
2. Burnett AL, Bivalacqua TJ. Priapsim Current Principles and Practice. Urol Clin North Am.2007;34:631–42. [PubMed]
3. Wagner MJ. PEER VII: Physician’s evaluation and educational review in emergency medicine.ACEP; 2006. (revised 2007).
4. Numan F, Cantasdemir M, Ozbayrak M, et al. Posttraumatic Nonischemic Priapism Treated with Autologous Blood Clot Embolization. J Sex Med. 2008;5:173–9. [PubMed]
5. Samm BJ, Dmochowski RR. Urologic emergencies. Postgrad Med. 1996;100:187–200. [PubMed]
6. Merritt AL, Haiman C, Henderson SO. Myth: Blood transfusion is effective for sickle-cell anemia associated priapism. CJEM. 2006 Mar;8:119–22. [PubMed]
7. Muneer A, Cellek S, Ralph DJ, et al. The investigation of putative agents, using an in vitro model, to prevent cavernosal smooth muscle dysfunction during low-flow priapism. BJU Int. 2008;102:988–92. [PubMed]
8. Burnett AL. Molecular pharmacotherapeutic targeting of PDE5 for preservation of penile health. J Andrology. 2008;29:3–14.
9. Filippone LM. Diagnosis: Paraphimosis. Emerg Med News. 2005 Sep 18;27:18.
10. Parker S.Circumcision. Surgical-tutor.org.uk – a free online surgical resource. Available at:http://www.surgical-tutor.org.uk/default-home.htm?system/hnep/circumcision.htm~rightAccessed June 23, 2008.
11. Little B, White M. Treatment options for paraphimosis. Int J Clin Pract. 2005;59:591–3.[PubMed]
12. DeVries CR, Miller AK, Packer MG. Reduction of paraphimosis with hyaluronidase. Urology.1996;48:464–5. [PubMed]
13. Kumar V, Javle P. Modified puncture technique for reduction of paraphimosis. Ann Roy Coll Surg. 2001;83:126–7.
14. Gonzalez FM, Sousa EMA, Parra ML. Sugar: treatment of choice in irreducible paraphimosis.Actas Urol Esp. 2001;25:393–5. [PubMed]
15. Houghton GR. The “iced-glove” method of treatment of paraphimosis. BJS. 1973;60:876–7.
16. Kapoor S. Testicular torsion: a race against time. Int J Clin Pract. 2008;62:821–7. [PubMed]
17. Shah BR, Lucchesi M. Atlas of Pediatric Emergency Medicine. New York, NY: McGraw Hill; 2006.
18. Gatti JM. Current management of the acute scrotum. Semin Pediatr Surg. 2007;16:58–63.[PubMed]
19. Galejs LE, Kass EJ. Diagnosis and treatment of the acute scrotum. Am Fam Physician.1999;59:817–24. [PubMed]
20. Kadish HA, Bolte RG. A retrospective review of pediatric patients with epididymitis, testicular torsion, and torsion of testicular appendages. Pediatrics. 1998;102:73–6. [PubMed]
21. Rabinowitz R. The importance of the cremasteric reflex in acute scrotal swelling in children. J Urol. 1984;132:89–90. [PubMed]
22. Ringdahl E, Teague L. Testicular torsion. Am Fam Physician. 2006;74:1739–46. [PubMed]
23. Coley B. The acute pediatric scrotum. Ultrasound Clinics. 2006;1:485–96.
24. Sonda PL, Wang S. Evaluation of male external genital diseases in the emergency room setting.Emer Med Clin North Am. 1988 Aug;6:473–486.
25. Akgur FM, Killinc K, Aktug T, et al. The effect of allopurinol pretreatment before detorting testicular torsion. J Urol. 1994;151:1715–17. [PubMed]
26. Silva AC, Ortiz V, Silva RA, et al. Effect of allopurinol on rat testicles morphology, submitted to ischaemia for spermatic cord torsion followed by reperfusion. Acta Cir Bras. 2005;20:468–72.[PubMed]
27. Abasiyanik A, Dagdonderen L. Beneficial effects of melatonin compared with allopurinol in experimental testicular torsion. J Pediatr Surg. 2004;39:1238–41. [PubMed]
28. Payabvash S, Salmasi AH, Kiumehr S, et al. Salutary effects of N-acetylcysteine on apoptotic damage in a rat model of testicular torsion. Urol Int. 2007;79:248–54. [PubMed]
29. Ozkan KU, Boran C, Kilinç M, et al. The effect of zinc aspartate pretreatment on ischemia-reperfusion injury and early changes of blood and tissue antioxidant enzyme activities after unilateral testicular torsion-detorsion. J Pediatr Surg. 2004;39:91–5. [PubMed]
30. Atik E, Görür S, Kiper AN. The effect of caffeic acid phenethyl ester (CAPE) on histopathological changes in testicular ischemia-reperfusion injury. Pharmocol Res. 2006;54:293–7.
31. Cohen MS. Current experience and management of Fournier’s gangrene. Lecture presented at: 78th Annual Meeting of the Am Urol Assoc; 1983.
32. Parks J.Buckeye Surgeon: Fournier’s Gangrene. Available at:http://ohiosurgery.blogspot.com/2008/04/fourniers-gangrene.html Accessed January 13, 2009.
33. Mindrup SR, Kealey G, Fallon B. Hyperbaric oxygen for the treatment of Fournier’s gangrene. J Urol. 2005;173:1975–7. [PubMed]


