| Author | Affiliation |
|---|---|
| Steven R. Offerman, MD | Kaiser Permanente South Sacramento, Department of Emergency Medicine, Sacramento, California |
| Adina S. Rauchwerger, MPH | Kaiser Permanente, Division of Research, Oakland, California |
| Daniel K. Nishijima, MD | University of California Davis School of Medicine, Davis, California |
| Dustin W. Ballard, MD | Kaiser Permanente San Rafael, Department of Emergency Medicine, San Rafael, California |
| Uli K. Chettipally, MD | Kaiser Permanente South San Francisco, Department of Emergency Medicine, San Francisco, California |
| David R. Vinson, MD | Kaiser Permanente Roseville, Department of Emergency Medicine, Roseville, California |
| Mary E. Reed, DrPH | Kaiser Permanente, Division of Research, Oakland, California |
| James F. Holmes, MD | University of California Davis School of Medicine, Davis, California |
ABSTRACT
Introduction:
The adoption of electronic medical records (EMRs) in emergency departments (EDs) has changed the way that healthcare information is collected, charted, and stored. A challenge for researchers is to determine how EMRs may be leveraged to facilitate study data collection efforts. Our objective is to describe the use of a unique data collection system leveraging EMR technology and to compare data entry error rates to traditional paper data collection.
Methods:
This was a retrospective review of data collection methods during a multicenter study of ED, anti-coagulated, head injury patients. On-shift physicians at 4 centers enrolled patients and prospectively completed data forms. These physicians had the option of completing a paper data form or an electronic “dotphrase” (DP) data form. A feature of our Epic®-based EMR is the ability to use DPs to assist in medical information entry. A DP is a preset template that may be inserted into the EMR when the physician types a period followed by a code phrase (in this case “.ichstudy”). Once the study DP was inserted at the bottom of the electronic ED note, it prompted enrolling physicians to answer study questions. Investigators then extracted data directly from the EMR.
Results:
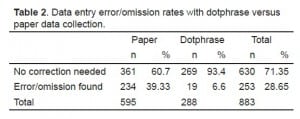
From July 2009 through December 2010, we enrolled 883 patients. DP data forms were used in 288 (32.6%; 95% confidence interval [CI] 29.5, 35.7%) cases and paper data forms in 595 (67.4%; 95% CI 64.3, 70.5%). Sixty-six (43.7%; 95% CI 35.8, 51.6%) of 151 physicians enrolling patients used DP data entry at least once. Using multivariate analysis, we found no association between physician age, gender, or tenure and DP use. Data entry errors were more likely on paper forms (234/595, 39.3%; 95% CI 35.4, 43.3%) than DP forms (19/288, 6.6%; 95% CI 3.7, 9.5%), difference in error rates 32.7% (95% CI 27.9, 37.6%, P < 0.001).
Conclusion:
DP data collection is a feasible means of data collection. DP data forms maintain all study data within the secure EMR environment, obviating the need to maintain and collect paper data forms. This innovation was embraced by many of our emergency physicians and resulted in lower data entry error rates.
INTRODUCTION
Electronic medical records (EMR) are being implemented by many United States (U.S.) healthcare systems. Conversion to EMR throughout the U.S. healthcare system is a stated goal of the federal government. New regulations and incentives are sure to increase implementation in the coming years.1,2 The use of EMRs in emergency departments (EDs) has fundamentally changed the way healthcare information is collected, recorded, and stored. Necessarily, this movement also impacts the way research data is collected.
Electronic data capture (EDC) refers to direct electronic entry of study data at the site of enrollment. While it is generally accepted that EDC should improve data integrity, decrease costs, and more rapidly transmit study data, implementation has been slow for multiple reasons.3 Some of the barriers noted previously have been cost, user acceptance, software installation, need for technical support, and regulatory requirements.3 Electronic data entry has been used previously by primary care research networks (practice-based research networks).4 However, the use of existing EMRs for research data collection has been noted as problematic as this data is rarely collected according to study protocol.5 A challenge for future researchers is to determine ways that EMRs may be leveraged to facilitate data collection efforts.
Our multi-center, collaborative emergency medicine (EM) research group recently employed a novel technique for prospective data collection that uses the Epic-based EMR (Epic Systems Corporation, Verona, Wisconsin) in use at our participating medical centers. Our objectives were to describe the use of this unique data collection method, the dotphrase (DP) data collection template, which used EMR technology and to compare its data entry error or omission rate versus a traditional paper data collection instrument.
METHODS
This is a retrospective review of data collection methods during the first 18 months of a multi-center study of ED head injury patients. Patients presenting to one of five study EDs with head injury and taking anti-coagulant medication (warfarin or clopidogrel) were eligible for inclusion. Treating ED physicians identified and enrolled eligible patients. These physicians then completed a prospective data form answering questions related to the primary study objectives. They had the option of completing a paper data collection form or an electronic DP data collection form inserted as a structured note template into the EMR at the end of their ED note. Physicians enrolling a patient (using either data collection form) were given a $10 coffee gift card as a token of appreciation for their participation.
Our hospital system uses an Epic®-based EMR similar to many other hospitals and healthcare systems in the United States. Kaiser Permanente’s EMR, powered by Epic, is the world’s largest privately owned EMR system, and securely hosts medical records for more than 8.6 million patients.6 A feature of this system is the ability of clinicians to use DPs to assist in medical information entry. A DP is a structured note template that may be easily inserted into the EMR. A template is populated into the data entry field when the physician types a period followed by a code phrase (in this case “.ichstudy”). We developed an EMR DP that included all of the questions relevant to our study and matched the questions on the paper data collection form. Once this study DP was inserted at the bottom of the electronic ED note, it prompted enrolling physicians to answer study questions before closing the note. The note writer simply uses the tab key to move between sections of the form to complete data entry. Importantly, the DP required physicians to respond to each question on the enrollment form, and offered pre-specified choices for most questions. Using the DP, it is difficult to omit answers although not impossible. Once the ED note (with the embedded data collection section) was completed, the note could easily be “routed” electronically to the study site primary investigator. Study investigators were then able to extract prospectively entered data directly from the EMR at a later time. DP data collection maintains all study data within the secure EMR environment and obviates the need to stock, collect, and store paper data forms.
We retrospectively collected data related to the use of DP versus paper data collection methods. For the purposes of this study analysis, we treated 2 of the participating EDs as a single study site because these departments are managed and staffed by the same emergency physician group (Site C). The study investigators collected data error and omission rates during the course of the study. We defined a data omission or error as any data field that was left empty or was unclear to research coordinators, requiring contact with the site investigator and/or clinician for clarification. Information regarding physician demographics and health plan tenure was abstracted from ED administrative records. Findings are reported using simple, descriptive statistics, with 95% confidence intervals (CI) provided where appropriate. We used multivariate logistic regression to examine the adjusted relationship between physician characteristics and use of the DP enrollment form.
This study was approved by the Kaiser Foundation Research Institute Investigational Review Board.
RESULTS
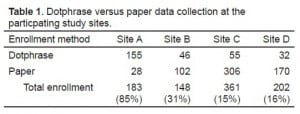
During the study period (July 1, 2009 through December 31, 2010) 883 patients were enrolled. DP data collection forms were used in 288 (32.6%; 95% CI 29.5, 35.7) cases and paper data forms used in 595 (67.4%; 95% CI 64.3, 70.5) cases. The prevalence of DP data collection use at the respective study centers was 84% (Site B), 29% (Site A), 15% (Site C), and 14% (Site D). There was no correlation between DP usage and overall enrollment rates at the different study sites (P > 0.05).
Of 151 physicians enrolling patients into the study, 66 (43.7 %; CI 35.8, 51.6) used DP data entry at least once, with 80.6% (Site A), 58.8% (Site B), 23.0% (Site C), and 41.7% (Site D) of physicians at the 4 study centers using the DP to enroll 1 or more patients. Using multivariate analysis, we found no significant association between physician age, gender, race, or tenure within the health system and DP use. The physician’s medical center was strongly associated with DP use (P < 0.0001). Two hundred and thirty-four of 595 paper data forms contained a data error or omission (39.3%; CI 35.4, 43.3),and 19 of 288 DP data collection templates contained data omissions or errors (6.6%; CI 3.7, 9.5). The difference in omissions or error rates was 32.7% (95% CI 27.9, 37.6%, P < 0.001) between DP and paper data collection forms. For data regarding enrollment methods at the participating study sites, see tables. We are not aware of any breach of protected health information (PHI) in data collected either by paper forms or by dotphrase templates.
LIMITATIONS
This was a purely observational study without intervention and control groups. Because physicians were allowed to choose between DP and paper data forms, bias is possible. Physicians who declined to accept this innovation might have made errors or omissions at a higher rate. We feel that the selection of data entry method was most influenced by familiarity with the EMR (different sites had used the EMR for varying lengths of time). We were unable to distinguish demographic differences between physicians using the DP and paper data forms. Total automation of DP variables into an analyzable database was not possible due to a lack of systems to support Direct Data Capture at the time of this study.
DISCUSSION
We present our experience with the use of a novel system of prospective data collection, the DP data collection template, which used our EMR for research purposes. Although DP use varied by medical center, we found that use of this data collection system was well accepted by many ED clinicians and yielded fewer missed data points. One barrier to the conduct of prospective research in emergency medicine is the difficulty associated with data collection that is frequently performed by ED staff. These clinicians may simply forget to enroll patients or may see study enrollment as an inconvenience that prevents them from completing study forms. Any system that makes data collection easier is likely to increase study enrollment.7 We feel that use of DP data collection may be easier for clinicians to access as there is no need to find a paper data form or return it to the primary study investigator. Clinicians can easily access the DP template within the EMR as they write progress notes, while patient details are fresh in memory. Additionally these electronic data forms never run out, alleviating the ongoing task of supplying paper data sheets by the study investigators or coordinator.
While we did not experience any breaches in PHI with either data collection source, another advantage to electronic DP data collection is the additional security associated with it. All data collection forms exist only within the hospital EMR that is password protected and accessible only to health system employees with proper security clearance. Once data forms are completed they are sent directly to the study investigator through the EMR system. This alleviates any need for security measures associated with paper data forms (e.g. locked files). These data collection forms also cannot be lost and do not need to be stored in a separate location during or after the completion of the study. This aspect of the EMR embedded data collection system is important as data security is a known barrier to use of EDC systems.3,8
We found that the incidence of missed data points was lower in those cases where the DP data collection instrument was used. Because the EMR system will not allow a note to be “accepted” (closed) until all template prompts have been addressed, note writers must answer all study questions. This substantially improves data collection integrity by reducing rate of data collection omissions. Therefore, it improves researcher efficiency as investigators and/or study coordinators have fewer missed data points to address.
Another important aspect of this system is that it uses existing EMR software for research purposes. Unlike other EDC systems employed in the past, there is no need for additional software or data security systems.3,8 Physicians also do not need to enter a secondary site for data entry as the DP is entered and remains within the EMR. Researchers whose health systems possess a functioning EMR can implement a similar data collection system almost immediately and with minimal training for site investigators. Additionally, incorporating a study specific DP template was quickly programmed into the EMR with no additional cost to the existing EMR infrastructure and with minimal information technology support. We found, using our Epic-based EMR, that it was easy to design data collection forms that closely resembled our paper instruments.
A possible advantage of an embedded EMR data collection format is the potential for development of direct data capture (DDC) systems in the future. Software could be developed that will directly extract study data from these EMR DP notes, thus alleviating the need for human data transfer from the EMR into central data storage software.
CONCLUSION
DP data collection is a feasible, efficient, and low cost means of study data collection. This innovation was embraced by many of our emergency physicians; however, adoption rates varied by medical center. We found lower error rates associated with DP data forms when compared with paper forms. DP technology used existing EMR infrastructure, required minimal technical support, and was up and running in a short time period. As a participant enrollment tool, a similar DP model could be easily replicated at another Epic-based EMR medical center.
Footnotes
Supervising Section Editor: Erik D. Barton, MD, MBA
Submission history: Submitted September 8, 2012; Accepted November 19, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.11.13400
Address for Correspondence: Steven R. Offerman, MD. Kaiser Permanente South Sacramento, Department of Emergency Medicine, 6600 Bruceville Road. Sacramento, CA 95823. Email: steve.offerman@gmail.com.
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none
REFERENCES
1. Trendwatch. The road to meaningful use: What it takes to implement electronic health record systems in hospitals. American Hospital Association. April 2010. Available at:http://www.aha.org/aha/trendwatch/2010/10apr-tw-HITmeanuse.pdf. Accessed August 23, 2011.
2. Jha AK, DeRoches CM, Campbell EG, et al. The use of electronic health records in U.S. hospitals. N Engl J Med. 2009;360:1628–38. [PubMed]
3. Welker JA. Implementation of electronic data capture systems. Comtemp Clin Trials.2007;28(3):329–36. [PubMed]
4. Kho A, Zafar A, Tierney W. Information technology in PBRNs: the Indiana University Medical Group Research Network (IUMG ResNet) experience. J Am Board Fam Med. 2007;20(2):196–203.[PubMed]
5. Pace WD, Staton EW. Electronic data collection options for practice-based research networks. Ann Fam Med. 2005;3(suppl 1):S21–29. [PMC free article] [PubMed]
6. Kahn R, Harris G. Kaiser Permanente completes electronic health record implementation. Mar 3, 2010. Available at:http://xnet.kp.org/newscenter/pressreleases/nat/2010/030310ehrcomplete.html. Accessed August 23, 2011.
7. Ross S, Grant A, Counsell C, et al. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156. [PubMed]
8. Brandt CA, Argraves S, Money R, et al. Informatics tools to improve clinical research study implementation. Contemp Clin Trials. 2006;27(2):112–122. [PubMed]