| Author | Affiliation |
|---|---|
| Chris Chee, DO | Kern Medical Center, Department of Emergency Medicine, Bakersfield, CA |
| Paul Walsh, MB, BCh, MSc | Kern Medical Center and Department of Internal Medicine, University of California, Los Angeles, CA |
| Sam Kuan, MB, BCh, BAO | Kern Medical Center, Department of Emergency Medicine, Bakersfield, CA |
| Juanito Cabangangan, MD | Kern Medical Center, Department of Emergency Medicine, Bakersfield, CA |
| Kian Azimian, MD | Kern Medical Center, Department of Emergency Medicine, Bakersfield, CA |
| Christopher Dong, MD | Kern Medical Center, Department of Emergency Medicine, Bakersfield, CA |
| Joshua Tobias, MD | Kern Medical Center, Department of Emergency Medicine, Bakersfield, CA |
| Stephen J. Rothenberg, PhD | Departamento de Ecología Humana, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (I.P.N), México |
ABSTRACT
Introduction:
To identify factors associated with culture-proven serious bacterial infection (SBI) and positive emergency department septic screening (EDSS) tests in children with bronchiolitis and to identify factors associated with the performance of EDSS.
Methods:
We reviewed an existing study database of patients with bronchiolitis. We defined a positive EDSS as urine with ≥10 WBC per high power field or cerebrospinal fluid (CSF) with ≥10 WBC per high power field (>25 WBC in neonates), or if organisms were identified on gram stain. We defined SBI as significant growth of an accepted pathogen in blood, urine or CSF. Our composite endpoint was positive if either of these was positive. The decision to perform testing was modeled using modified Poisson regression; the presence of the combined outcome was modeled using logistic regression modified for rare events.
Results:
We studied 640 children. Testing was performed in 199/640 (31.1%). These tended to be younger than two months RR 2.69 (95% CI 2.11, 3.44), febrile RR 2.01 (95% CI 1.58, 2.55), more dehydrated RR 1.50 (95% CI 1.28, 1.75) and had more severe chest wall retractions RR 1.54 (95% CI 1.22, 1.94). Only 11/640(1.7%) had a positive EDSS or SBI. Younger age (OR 0.67 per month; 95% CI 0.45, 0.99) and a negative RSV antigen test (OR 6.22; 95% CI 1.30, 29.85) were associated with the composite endpoint.
Conclusion:
Testing was more likely to be performed in children younger than two months of age, and in those who were febrile, dehydrated, and had more severe chest wall retractions. A positive EDSS or SBI was rare occurring in younger infants with non-RSV bronchiolitis.
INTRODUCTION
The detection of respiratory syncytial virus (RSV) decreases the probability of a concurrent bacterial illness in infants with fever without source.1–3 The clinical presentation of RSV is highly variable. It causes more than half of all cases of bronchiolitis.4,5 Other viruses are responsible for almost all of the remainder.4,6–9
Bronchiolitis itself is a clinically recognizable viral illness; consequently, a low prevalence of culture-proven serious bacterial illness (SBI) would be anticipated.10–12 This raises the question whether bronchiolitis caused by RSV as distinct from bronchiolitis caused by other viruses has a different prevalence of bacterial co-infection and therefore warrants a different emergency department (ED) evaluation.
The primary purpose of this study was to identify those clinical factors most likely associated with SBI and positive ED screening tests in children with bronchiolitis. The secondary purpose was to identify factors associated with obtaining these screening tests and cultures.
METHODS
Underlying assumptions
We made the following assumptions about emergency physicians’ (EP) risk tolerance: (1) a positive screening test for SBI would prompt a change in management regardless of subsequent outcomes (since these cannot be known ahead of time); (2) EPs would not want to miss an infant who had a positive culture despite a (false) negative screening test.
We made the following assailable but unavoidable assumptions about bacterial illness in these patients. Firstly, we assumed that if both screening tests and cultures were negative that SBI was absent. Secondly, we assumed if no screening was performed without any adverse clinical outcomes three days post-ED visit, then SBI was absent. We anticipated that the outcomes we were seeking would be sufficiently rare that our primary analysis would require a combined endpoint rather than separate analyses of several single endpoints.
Study Design
Our Institutional Review Board approved this study. We conducted a retrospective review using existing patient records and an existing database of ED patients prospectively enrolled into an RCT over three bronchiolitis seasons.5
Setting and Population
The parent study was conducted at two centers; this study used data only from the primary study site. The primary study site was a county teaching hospital ED with an annual census of 53,000 (23% children less than 14 years) serving a mixed urban, suburban, and rural population.
Bronchiolitis was defined as clinical evidence of lower airway obstruction (physical findings of wheezing and chest wall retractions) following an upper respiratory tract infection. The eighteenth month of life was chosen as the upper age limit. This represents a compromise between competing views as to an appropriate definition of the diagnosis.5,10
Neonate was defined as age up to 28 days. Dehydration and retraction severity were each described using scales from a validated bronchiolitis severity assessment tool.13 The inter rater reliability of these individual variables and that of the severity of illness tool been described elsewhere.14 Fever was defined as rectal temperature ≥38.0°C.
Patients were enrolled consecutively. Those with bronchiolitis so mild as to not require bronchodilator treatment and those with illness so severe as to require immediate intubation were excluded. These criteria reflect the design of the parent study, a randomized controlled trial of bronchodilators in bronchiolitis. We restricted this study to patients from that study to ensure the quality of data collected. Patients who received bronchodilators in the ED prior to screening for the parent study were excluded from that study and also from this. Although the patients included in this study reflect the parent study design, it is difficult to imagine an EP prescribing antibiotics in infants with bronchiolitis so mild as to not be eligible for the original trial, or withholding them in patients intubated for bronchiolitis.
Patients were recruited between November 1, 2003 and May 1, 2006. Potentially eligible children were identified by trained research assistants, residents and faculty. Diagnosis was made by an attending physician or midlevel provider. Clinical findings, including prior antibiotic and steroid use, were recorded on a specifically designed data collection. Laboratory testing was ordered at the physician’s discretion.
Laboratory tests
We performed RSV antigen detection on nasal aspirates samples using the BD Directigen™ RSV Test Kit (Becton, Dickinson and Company, Franklin Lakes, NJ).
We performed urinalysis and cultures on catheterized specimens. Urinalysis was performed with an Iris iQ200 Automated Urine Microscopy Analyzer (Iris Diagnostics, Chatsworth, CA). Urine culture was performed using the calibrated-loop method on well-mixed, uncentrifuged specimens on blood agar and MacConkey agar plates. Low colony count plates (recommended if the child has received antibiotics prior to the sample being obtained) were used if specified by the physician. Results were reported after 48 hours of incubation. Cerebrospinal fluid (CSF) specimens were processed for protein, glucose, red and white blood cell counts (RBC, WBC), gram stain (GS) and cultures. Blood cultures were processed using the BacT/ALERT system (bioMérieux, Marcy l’Etoile, France), and their results were generally available after 48 hours.
Study Protocol
We obtained clinical data from the study database. Testing results were obtained from the hospital laboratory database. The clinical record was reviewed by first and second authors to determine whether positive cultures were treated by the physician.
Outcome measures:
An ED septic screen (EDSS) was considered positive if urine or CSF analysis showed ≥10 WBC per high power field (>25 WBC in neonates) or organisms were identified on gram stain regardless of subsequent culture being positive or negative. CSF WBC counts were corrected assuming one additional WBC to be normal for every 500 RBCs present.
We defined culture-proven SBI as a significant growth of a known bacterial pathogen in urine (10,000 or more colony-forming units per milliliter of a single pathogenic organism on a catheterized specimen), CSF or blood regardless of initial urine or CSF WBC results. When more than one organism was detected in a urine culture the specimen was considered contaminated. We considered blood cultures contaminated if they grew organisms commonly not accepted as pathogens (such as coagulase-negative staphylococcus, diphtheroids, and alpha- or gamma-hemolytic streptococcus).
We created a composite endpoint comprised of either a positive EDSS or a culture-proven SBI, or both. Patients who did not have laboratory tests and were alive 72 hours post-ED discharge (as assessed by telephone follow-up) were assumed not to have had either an SBI or the composite endpoint. In cases where both telephone and written follow-up failed, and a review of hospital medical records showed no further contact with the patient, the county coroner’s records were used to determine vital status.
We did not address the possibility of pneumonia as an SBI because of the inability to differentiate a viral from a bacterial cause on chest x-ray.
Data analysis
We compared the prevalence of outcomes between RSV-positive and RSV-negative groups using Fisher’s exact test for categorical data. We analyzed the importance of age, gender, presence of fever, clinical exam findings, and prior community antibiotic use on outcomes using logistic regression multivariate analysis. A modified multivariate Poisson analysis15 was performed to determine factors associated with having an EDSS performed or not performed. We used logistic regression modified for rare events to account for biased standard logistic regression estimates in the case of a rare outcome.16,17 We performed model diagnostics on the standard logistic regression model as described in other medical literature using this technique.18 Tests reached statistical significance atp ≤ .05. We used Stata 9.2 statistical software, (Statacorp LP, College Station TX), for all analyses.
RESULTS
Data were available for 640 patients. RSV antigen testing was performed in 608 (95%) of these 640. The ED ordered RSV testing in 553 cases, the admitting pediatric service ordered it in 55. Three hundred thirty-four patients (54.9%) tested positive for RSV antigen, 271 (44.6%) tested negative and in 3 (0.5%) results were equivocal.
The median age of patients was 4.8 months. Patients’ clinical and demographic characteristics are grouped by RSV status in Table 1.
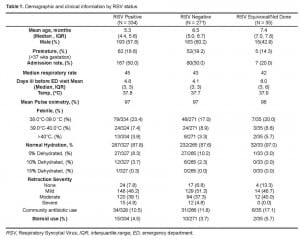
ED testing to rule out bacterial illness was performed in 199/640 (31.1%) of patients. There were differences between children in whom physicians did and did not perform testing for bacterial illness. Those investigated were more likely to be younger than two months RR 2.69 (95% CI 2.11, 3.44), be febrile RR 2.01 (95% CI 1.58, 2.55), be more dehydrated RR 1.50 (95% CI 1.28, 1.75), and have more severe chest wall retractions RR 1.54 (95% CI 1.22, 1.94). RSV antigen results did not dissuade physicians from testing, RR 0.82 (95%CI 0.65, 1.04).
Although 14 patients were treated as having a positive EDSS or SBI by their treating physicians, our study definitions led us to discount three cases. These are shown in Table 2. Using study definitions, the combined endpoint of a positive septic screen, culture-proven SBI, or both occurred in 2/334 (0.6%, 95% CI 0.1% to 2.1%) and 9 of 271 (3.3%, 95% CI 1.5% to 6.2%) in RSV and non-RSV bronchiolitis, respectively (two sided Fisher’s exact, p=.02).
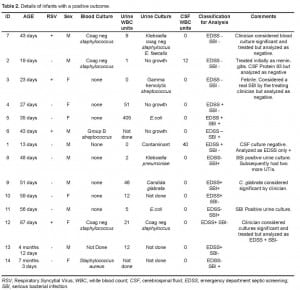
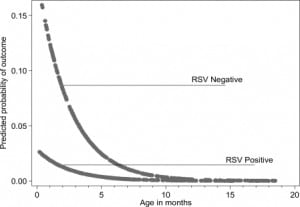
Using logistic regression modified for rare events, younger age (OR 0.67 per month; 95% CI 0.45, 0.99) and a negative RSV antigen test (OR 6.22; 95% CI 1.30, 29.85) were associated with the composite endpoint. These results are presented graphically in the Figure. Using ordinary logistic regression, age (OR 0.63 per month; 95% CI 0.43, 0.94) and a negative RSV antigen test (OR 7.77; 95%CI 1.61, 37.56) were associated with the primary or composite endpoint. The results were similar (age OR 0.57, RSV negative OR 4.81) when we analyzed the treating physicians’ clinical impressions rather than study definitions of which infants had a positive EDSS or SBI.
The results were similar for the individual components (EDSS and SBI) of the endpoint. The prevalence of a positive EDSS was 7/605(1.1% 95% CI 0.4%, 2.2%); 1 of 334 (0.3%, 95% CI 0.0%, 1.7%) in the RSV group and 6 of 271 (2.2%, 95% CI 0.8%, 4.8%) in non-RSV bronchiolitis (two sided Fisher’s exact, p=.049). Prevalence of a culture-proven SBI was 6/640 (0.9% 95% CI 0.3% to 2.0%). One of 334 (0.3%; 95% CI 0.0% to 1.7%) had RSV-positive and 5 of 271 (1.8%, 95% CI 0.06% to 4.3%) had RSV-negative bronchiolitis, (two-sided Fishers exact p=0.095).
Of the individual EDSS components, blood cultures were the most frequently obtained. However, urinary studies were the most likely to yield positive findings. Urine microscopy was performed in 95/640 (14.8%), urine cultures in 71/640 (11.0%), blood cultures in 183/640 (28.6%) and lumbar punctures in 10/640 (1.5%). Six out of 95 (6.3%) patients had >10 WBC in on urine microscopy, 2/10 (20%) had >10 WBC (corrected) in the CSF, and 7/71 (9.9%) had a positive urine culture. Twenty-one of 183 blood cultures grew organisms; of these, only two were recognized pathogens.
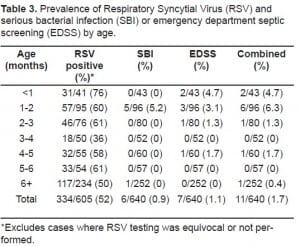
Prior antibiotic use did not seem to alter outcome; low colony count plates were not used in some children who had had prior antibiotics. The prevalence of SBI and EDSS in each age group is shown in Table 3.
DISCUSSION
In children up to their eighteenth month of life with bronchiolitis, an EDSS was more likely to be performed in those who were less than two months old, febrile, dehydrated, and had more severe chest wall retractions; in short, those who appeared sicker. This evaluation was more likely to be positive in those who were younger and had a negative RSV antigen test. Once the decision to screen was made, neither fever nor the height of the fever was associated with SBI or positive EDSS. The paucity of positive outcomes despite relatively large sample size resulted in broad confidence intervals for our estimates of these effects.
Studies of febrile children have shown that when a viral etiology can be demonstrated, typically by RSV or influenza antigen testing, concurrent bacterial infection is rare.1–3,11,19 Despite this, RSV status did not significantly affect the decision to obtain these tests in our study (RR 0.82 95% CI 0.65–1.04). Perhaps with larger numbers this trend would have reached significance. Similarly, in the presence of a recognizable viral illness concurrent bacterial infection is also unlikely.11 We anticipated, and found, very low rates of culture- proven bacterial illness (0.9%) or EDSS (1.1%) in infants with bronchiolitis. One might reasonably expect, therefore, that RSV antigen testing would add little information in the risk stratification of these children. But it did, with a negative RSV test increasing the odds of EDSS or SBI by 6.22.
Why the prevalence of either a positive EDSS or SBI should differ in RSV as distinct from non-RSV bronchiolitis is a matter of conjecture. Although clinically indistinguishable, the inflammatory profile of RSV bronchiolitis differs from that of other viruses.20–23
A weakness in this study is that EDSS was variable in its components. Some children had blood, CSF and urine cultured, and some, typically older infants, only had urine cultured. This is inherent in the retrospective use of even prospectively collected research data. Even if a purely prospective design were employed, it would not be ethical to subject children to investigations that the treating clinician did not believe were indicated.
A related limitation is the possibility of a missed SBI in those not screened and who did not have an adverse outcome within three days of enrollment in the parent study. The most likely source in these cases would be urinary, and the most likely pathogen Escherichia coli, (E. coli). Where the infection is confined to the urinary tract it is possible that some infants would have cleared the infection spontaneously. Sepsis in conjunction with UTI occurred in 31% of neonates, 21% of infants aged 1 to 2 months, 14% of those aged two to three months, and 5.5% of infants over three months of age in one series.24 Mortality rates as high as 45% to 73%, depending on the underlying prognosis, have been reported in children growing E. coli in blood cultures.25 As there was only one death within 30 days of enrollment (in a severely co-morbid ex-premature infant), a clinically important miss rate for SBI in unscreened patients seems unlikely.
Another limitation is the use of RSV antigen rather than PCR testing. The sensitivity and specificity of the test we used is modest, 78 % and 67% respectively.26 Whether and how PCR testing would have changed our results is speculative.
We chose our combined endpoint as the primary outcome for several reasons. Culture- negative SBI can occur, with diagnosis hinging on screening tests and clinical judgment. Our necessarily practical definition of EDSS in this study included only tests with a turnaround time short enough to impact ED management. Dropping “screen positive culture negative” cases does not help an EP who cannot know beforehand what cultures will grow.
Including screening tests alone would be incomplete. Although infants with urinary WBC<10 are less likely to have a urinary tract infection,27,28 culturing is nonetheless recommended for all urine specimens obtained in this age group.29 While CSF pleocytosis is suggestive, its absence in a neonate does not preclude meningitis.30 Consequently, we felt that neither culture nor screening tests alone were a satisfactory outcome for emergency medicine.
Finally, when studying rare events, using a sensible combined outcome measure may allow insights to be obtained with smaller sample sizes than would otherwise be possible. The rarity of our outcome means that despite a sample size of 640 patients our confidence intervals are wide.
Our study definitions sometimes conflicted with the treating physicians’ clinical impression of a coexisting bacterial illness. Two neonates who were treated based on CSF results were ultimately felt not to have an SBI. We categorized one of these cases as having a positive EDSS but negative SBI. One case was treated by the clinician as having a real UTI based on urine culture of gamma hemolyticstreptococcus. We analyzed a case of Candida glabrata as having an EDSS and SBI in one patient who had pyuria. Though C. glabrata is often nosocomially acquired, it can be considered a non-trivial finding; this patient was treated.31 Some have questioned whether urinary bacterial colony counts less than 50,000 in the presence of low urine WBC are significant. We had only one case where this may have been an issue; however, this infant has had two subsequent UTIs. While reluctant to discount the judgment of the treating pediatrician, we felt obliged to analyze the data according to our study definitions. Reassuringly, when we analyzed the data based on the physicians’ clinical impressions the results were similar.
We were surprised that community antibiotic usage did not appear to affect the results. This may in part be because of the low prevalence of their use (11%). We were also surprised that blood cultures were ordered more frequently than urine cultures. They appear to have been ordered reflexively as part of the admission process in some cases.
Our overall prevalence of SBI was lower than some previous studies, which report a prevalence of SBI in bronchiolitis of 11.4%.1 In RSV bronchiolitis specifically, the prevalence rates range from none to 7.0 %.1,32 Children with RSV negative bronchiolitis have also been found by most though not all others to have a higher prevalence of SBI.1,34–36 These studies excluded afebrile infants. We found that while the presence of fever influenced the decision to work up an infant, once that decision was made, neither the presence nor height of the fever was associated with a concurrent bacterial illness. Had we excluded infants who were afebrile on presentation we would not have been able to demonstrate this point. This approach also allowed us to capture the practice of experienced clinicians who occasionally perform an EDSS in infants without a fever yet look unwell.
Unlike prior work2,31,34–36we included children older than three months of age; this allowed us to prove the importance of age as a predictor of concurrent bacterial infection.
There are some differences between our findings and those of others that bear examination. In Liebelt et al 211 infants aged 90 days or younger with clinical bronchiolitis, no case of SBI was found. Of note, 82% of that study’s patients had RSV-positive bronchiolitis.33 Given that SBI is more likely in RSV negative patients and the CIs for 0/38 overlap with our findings, this apparent difference is likely artifactual. Antonow et al studied 282 infants less than two months of age with clinical bronchiolitis and found a SBI prevalence of 1.8%. In that study two-thirds of patients were febrile and RSV rate was 83 % (34). In our study, 32% were febrile on ED presentation (42% febrile in Liebelt and 65% in Antonow) and 55% were RSV-positive.
Most children, even the very young, will have neither a positive EDSS nor an SBI. Among those who do, a UTI is the most common diagnosis.1,2,19,32,36 For individual patients, clinical judgment, which may be more cost effective than using “clinical rules,”37 remains necessary. This study provides a measure of the underlying prevalence to help inform that judgment. We have not attempted to provide specific thresholds at which EDSS may be withheld. Instead we have provided our results in a way that we believe informs individual physicians’ risk tolerance for what is a rare but important outcome.
Future work establishing the prevalence of concurrent bacterial lung infection in children with bronchiolitis is needed.
CONCLUSION
In those children with bronchiolitis in whom ED testing for bacterial illness was performed, younger age and a negative RSV antigen test was associated with a positive EDSS or SBI.
Footnotes
Supervising Section Editor: Supervising Section Editor: Judith R Klein, MD
Submission history: Submitted March 14, 2009; Revision Received June 23, 2009; Accepted July 13, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Paul Walsh MB, BCh, Department of Emergency Medicine, 1830 Flower St.., Bakersfield, CA 93305
Email: yousentWHOhome@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Levine DA, Platt SL, Dayan PS, et al. Risk of Serious Bacterial Infection in Young Febrile Infants with Respiratory Syncytial Virus Infections. Pediatrics. 2004;113(6):1728–34. [PubMed]
2. Titus MO, Wright SW. Prevalence of Serious Bacterial Infections in Febrile Infants With Respiratory Syncytial Virus Infection. Pediatrics. 2003;112(2):282–4. [PubMed]
3. Antonow JA, Byington CL, Liebelt EL, et al. Use of Respiratory Syncytial Virus Testing Could Safely Eliminate Many Sepsis Evaluations. Arch Pediatr Adolesc Med. 1999;153(12):1310. [PubMed]
4. Mansbach JM, McAdam AJ, Clark S, et al. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med. 2008;15(2):111–8. [PubMed]
5. Walsh P, Caldwell J, McQuillan KK, et al. Comparison of nebulized epinephrine to albuterol in bronchiolitis. Acad Emerg Med. 2008;15(4):305–13. [PMC free article] [PubMed]
6. Beem M, Wright FH, Fasan DM, et al. Observations on the etiology of acute bronchiolitis in infants.J Pediatr. 1962;61:864–9. [PubMed]
7. Garofalo RP, Hintz KH, Hill V, et al. A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J Med Virol.2005;75(2):282–9. [PubMed]
8. Korppi M, Kotaniemi-Syrjanen A, Waris M, et al. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23(11):995–9.[PubMed]
9. Ordas J, Boga JA, varez-Arguelles M, et al. Role of Metapneumovirus in Viral Respiratory Infections in Young Children. J Clin Microbiol. 2006;44(8):2739–42. [PMC free article] [PubMed]
10. Welliver JR, Welliver RC. Bronchiolitis. Pediatrics in Review. 1993;14(4):134–9. [PubMed]
11. Byington CL, Enriquez FR, Hoff C, et al. Serious Bacterial Infections in Febrile Infants 1 to 90 Days Old With and Without Viral Infections. Pediatrics. 2004;113(6):1662–6. [PubMed]
12. Greenes DS, Harper MB. Low risk of bacteremia in febrile children with recognizable viral syndromes. Pediatr Infect Dis J. 1999;18(3):258. [PubMed]
13. Walsh P, Rothenberg SJ, O’Doherty S, et al. A validated clinical model to predict the need for admission and length of stay in children with acute bronchiolitis. Eur J Emerg Med. 2004;11(5):265–72. [PubMed]
14. Walsh P, Gonzales A, Satar A, et al. The Interrater Reliability of a Validated Bronchiolitis Severity Assessment Tool. Pediatric Emergency Care. 2006;22(5):316. [PubMed]
15. Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data.American Journal of Epidemiology. 2004;159(7):702. [PubMed]
16. King G, Zeng L. Explaining Rare Events in International Relations. International Organization.2003;55(03):693–715.
17. King G, Zeng L. Logistic Regression in Rare Events Data. Political Analysis. 2001;9(2):137–63.
18. McQuirter JL, Rothenberg SJ, Dinkins GA, et al. Change in Blood Lead Concentration up to 1 Year after a Gunshot Wound with a Retained Bullet. American Journal of Epidemiology.2004;159(7):683–92. [PubMed]
19. Kuppermann N, Purcell K, Fergie J. Respiratory Syncytial Virus Infection and the Risk of Serious Bacterial Infections. Arch Pediatr Adolesc Med. 2002;156(10):1055–6. [PubMed]
20. Guerrero-Plata A, Casola A, Garofalo RP. Human Metapneumovirus Induces a Profile of Lung Cytokines Distinct from That of Respiratory Syncytial Virus. J Virol. 2005;79(23):14992–7.[PMC free article] [PubMed]
21. Pinto RA, Arredondo SM, Bono MR, et al. T Helper 1/T Helper 2 Cytokine Imbalance in Respiratory Syncytial Virus Infection Is Associated With Increased Endogenous Plasma Cortisol.Pediatrics. 2006;117(5):e878–e86. [PubMed]
22. Laham FR, Israele V, Casellas JM, et al. Differential Production of Inflammatory Cytokines in Primary Infection with Human Metapneumovirus and with Other Common Respiratory Viruses of Infancy. The Journal of Infectious Diseases. 2004;189(11):2047–56. [PubMed]
23. Welliver TP, Garofalo RP, Hosakote Y, et al. Severe Human Lower Respiratory Tract Illness Caused by Respiratory Syncytial Virus and Influenza Virus Is Characterized by the Absence of Pulmonary Cytotoxic Lymphocyte Responses. The Journal of Infectious Diseases.2007;195(8):1126–36. [PubMed]
24. Ginsburg CN, McCracken GH. Urinary Tract Infections in Young Infants. Pediatrics.1982;69(4):409–12. [PubMed]
25. DuPont HL, Spink WW. Infections due to Gram-negative Organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958–1966. Medicine. 1969;48:307–22. [PubMed]
26. Walsh P, Cabanangan J, Lona L, et al. Performance of a Rapid Respiratory Syncytial Virus Test in the Emergency Department. Academic Emergency Medicine. 2009;16(4):S103.
27. Lin D, Huang F, Chiu N, et al. Comparison of hemocytometer leukocyte counts and standard urinalyses for predicting urinary tract infections in febrile infants. Pediatr Infect Dis J.2000;19(3):223. [PubMed]
28. Hoberman A, Wald ER, Reynolds EA, et al. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304. [PubMed]
29. Shaw KN, McGowan KL, Gorelick MH, et al. Screening for Urinary Tract Infection in Infants in the Emergency Department: Which Test Is Best? Pediatrics. 1998;101(6):e1. [PubMed]
30. Garges HP, Moody MA, Cotten CM, et al. Neonatal Meningitis: What Is the Correlation Among Cerebrospinal Fluid Cultures, Blood Cultures, and Cerebrospinal Fluid Parameters? Pediatrics.2006;117(4):1094–100. [PubMed]
31. Vazquez JA, Dembry LM, Sanchez V, et al. Nosocomial Candida glabrata Colonization: an Epidemiologic Study. J Clin Microbiol. 1998;36(2):421–6. [PMC free article] [PubMed]
32. Purcell K, Fergie J. Concurrent Serious Bacterial Infections in 2396 Infants and Children Hospitalized With Respiratory Syncytial Virus Lower Respiratory Tract Infections. Arch Pediatr Adolesc Med. 2002;156(4):322–4. [PubMed]
33. Liebelt EL, Qi K, Harvey K. Diagnostic Testing for Serious Bacterial Infections in Infants Aged 90 Days or Younger With Bronchiolitis. Arch Pediatr Adolesc Med. 1999;153(5):525–30. [PubMed]
34. Antonow JA, Hansen K, McKinstry CA, et al. Sepsis evaluations in hospitalized infants with bronchiolitis. Pediatr Infect Dis J. 1998;17(3):231–6. [PubMed]
35. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053. [PubMed]
36. Kuppermann N, Bank DE, Walton EA, et al. Risks for bacteremia and urinary tract infections in young febrile children with bronchiolitis. Arch Pediatr Adolesc Med. 1997;151(12):1207–14.[PubMed]
37. Pantell RH, Newman TB, Bernzweig J, et al. Management and Outcomes of Care of Fever in Early Infancy. JAMA. 2004;291(10):1203–12. [PubMed]


