| Author | Affiliation |
|---|---|
| Jamie A. Jenkins, MD | Stanford University/Kaiser Permanente Emergency Medicine Residency Program, Division of Emergency Medicine, Department of Surgery, Palo Alto, California |
| Laleh Gharahbaghian, MD | Stanford University Medical Center Division of Emergency Medicine, Department of Surgery, Emergency Ultrasound Fellowship, Palo Alto, California |
| Stephanie J. Doniger, MD | Children’s Hospital and Research Center, Oakland Division of Emergency Medicine, Oakland, California |
| Scott Bradley, MD | Stanford University/Kaiser Permanente Emergency Medicine Residency Program, Division of Emergency Medicine, Department of Surgery, Palo Alto, California |
| Steve Crandall, MD | Stanford University School of Medicine Division of Emergency Medicine, Department of Surgery, Palo Alto, California |
| David A. Spain, MD | Stanford University, Trauma/Critical Care Surgery, Department of Surgery, Palo Alto, California |
| Sarah R. Williams, MD | Stanford University/Kaiser Permanente Emergency Medicine Residency Program, Stanford Emergency Medicine Ultrasound Program and Fellowship Division of Emergency Medicine, Department of Surgery, Palo Alto, California |
ABSTRACT
Introduction:
Thoracostomy tubes (TT) are commonly placed in the management of surgical, emergency, and trauma patients and chest radiographs (CXR) and computed tomography (CT) are performed to confirm placement. Ultrasound (US) has not previously been used as a means to confirm intrathoracic placement of chest tubes. This study involves a novel application of US to demonstrate chest tubes passing through the pleural line, thus confirming intrathoracic placement.
Methods:
This was an observational proof-of-concept study using a convenience sample of patients with TTs at a tertiary-care university hospital. Bedside US was performed by the primary investigator using first the low-frequency (5–1 MHz) followed by the high-frequency (10–5 MHz) transducers, in both 2-dimensional gray-scale and M-modes in a uniform manner. The TTs were identified in transverse and longitudinal views by starting at the skin entry point and scanning to where the TT passed the pleural line, entering the intrathoracic region. All US images were reviewed by US fellowship-trained emergency physicians. CXRs and CTs were used as the standard for confirmation of TT placement.
Results:
Seventeen patients with a total of 21 TTs were enrolled. TTs were visualized entering the intrathoracic space in 100% of cases. They were subjectively best visualized with the high-frequency (10–5 MHz) linear transducer. Sixteen TTs were evaluated using M-mode. TTs produced a distinct pattern on M-mode.
Conclusion:
Bedside US can visualize the TT and its entrance into the thoracic cavity and it can distinguish it from the pleural line by a characteristic M-mode pattern. This is best visualized with the high-frequency (10–5 MHz) linear transducer.
INTRODUCTION
The incidence of chest trauma is estimated at 12 persons per million of population per day.1 Of those, only 5% to 10% require thoracic surgery; the majority can be adequately managed medically, at times with thoracostomy tube (TT) or mechanical ventilation.1 A recent study showed that TTs are required in 25% of patients after major chest trauma.2 TTs have been shown to be malpositioned in up to 20% of cases with increasing numbers seen at teaching hospitals and in emergent settings.1 In order to identify proper TT placement, the American College of Surgeons recommends chest radiographs (CXR) as the initial imaging modality of choice.3 This is controversial since computed tomography (CT) of the chest may be better in determining TT malpositioning1,3,4 and have been determined to be the gold standard. However, both techniques expose the patient to radiation and require further time, resources, and expense. This is especially true in the intensive care unit (ICU), where patients are exposed to daily CXRs, and in the emergency department (ED), where multiple critical patients can present simultaneously. If TT repositioning is required, the patient is exposed to additional pain, more invasive procedures, and increased risk of infection.5–7 Associated complications include empyemas, tension pneumothorax, lung contusions, and vascular injury.1,3–8
Ultrasound (US) has become an accepted imaging modality in the ED and ICU. It is a proven bedside tool that evaluates numerous disease processes and aids procedure guidance without the risk of radiation exposure. Emergency physicians have demonstrated proficiency in a wide range of US applications.9–14Emergency physician visualization of the pleura by bedside US is already an accepted part of the eFAST exam.7,15–29 If the emergency physician can use bedside US in real time to visualize the chest tube going through the pleural line into the chest cavity, placement within the pleural space can be confirmed and the rate of chest tube malpositioning can be decreased.
It is important to optimize transducer choice to obtain the highest quality images for a given anatomic region.19 The lung and pleural line are best evaluated with the high-frequency linear transducer, which is ideal for delineation of superficial structures. The deeper thoracic and intra-abdominal regions are best evaluated with lower frequency transducers. Both transducer types are used for the eFAST scan: high frequency to evaluate for pneumothorax and low frequency to evaluate for intraperitoneal fluid and hemothorax.30 It is with this logic that we chose to use both high- and low-frequency probes in the study.
Due to the high rate and complications of malpositioned TTs, our primary objective was to evaluate whether bedside US can evaluate TT positioning within the pleural space and to define the best technique for this new US application.
METHODS
This observational proof-of-concept study approved by the institutional review board evaluated a convenience sample of consented adult patients with TTs from the ED and surgical floors at a tertiary care level one trauma center. Patients were identified by consultation with the emergency physicians who were part of thoracic and general surgical services on days where the sonographers were available. Those excluded were children, pregnant women, hospital employees and patients who declined to participate in the study.
The Sonosite M-Turbo (Bothell, Washington), with both the linear 10-5 MHz and phased array 5-1 MHz transducers were used for the study. Transducers and skin were disinfected adequately prior to performing the US. Sterile surgical lubricant was used at all times. Patient demographics (age and gender), reason for TT placement, service that placed the thoracostomy tube, presence and type of fluid in the TT (serosanguineous, air), and best US technique were collected and recorded by the sonographers on a common data sheet. All USs were performed anywhere from 15 minutes to 2 days after confirmatory CXRs were obtained to maintain current standard of care; however, the researchers were not privy to the results of the CXRs results nor did they view the CXRs prior to performing the US. The sonographers were composed of 3 fellowship-trained US faculty, 1 emergency US fellow, and 2 emergency medicine (EM) senior residents who were trained in emergency US through residency education. The primary investigator was present and involved in scanning during all image and data acquisitions to maintain standard of data collection.
The TTs were uniformly identified first in transverse and then longitudinal views by placing the linear transducer at the skin entry point and scanning to the point at which the TT penetrated the pleural line (Figure 1). The process was repeated with the low-frequency transducer. We alternated which transducer we used first in order not to affect the perceptions of the sonographers. Images were saved as still pictures and clips using 2-dimensional gray scale.
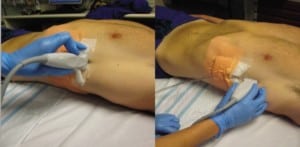
M-mode (“motion” mode) is a presentation of the temporal changes in echoes in which the depth of echo-producing interfaces is displayed along one axis and time is displayed along the second axis, recording motion of the interfaces toward and away from the transducer. Its applicability is expanding to encompass the evaluation of subcutaneous structures, including ribs.19 After the third consented subject we noticed that M-mode imaging provided a distinct image that could help in TT identification; this was used with all subjects thereafter.
The TT was considered to be within the pleural space by the sonographer if it was seen entering the pleural space in both perpendicular and parallel views. Collected images were reviewed by EM US fellowship-trained faculty for quality assurance and for confirmation of the TT placement. This was then compared to CXR and, if available a CT. The research team discussed which modality and technique were best for image acquisition and interpretation. There were no disagreements among the team.
RESULTS
Seventeen patients were enrolled, 4 of whom had 2 TTs, totaling 21 TTs evaluated. One TT was placed in the ED, the remaining were placed by inpatient surgical services. Patients’ demographic data, indication for TT, and chest tube sizes (ranging from 8.5 to 14 French pigtail tubes up to 20 to 32 French standard TTs) are illustrated in the Table. All TTs were determined to be in the pleural space by the sonographers, which were confirmed by CXR in all patients (the standard of care) or CT in 9 of the 21 TTs (the gold standard). Both transducer types visualized the TT. The images where reviewed by the US faculty and it was determined that the linear transducer produced images that were subjectively better in delineating the subcutaneous tissue from the TT, pleural line, and adjacent rib. Therefore, the linear transducer was better able to identify the TT position within the chest wall and at entrance into the thoracic cavity. After the TT entered the intrathoracic cavity, it disappeared from visualization except in cases when there was persistent fluid evident beneath the pleural line. In those cases, the TT was visualized within the fluid but disappeared from visualization when deep to the fluid.
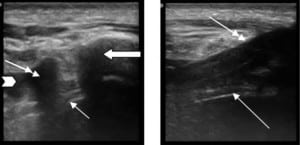
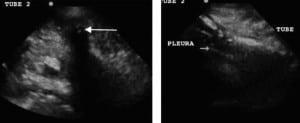
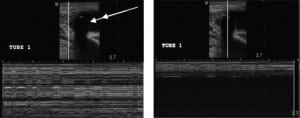
The US characteristics of the TTs are illustrated in Figures 2 and and3.3. With M-mode, the TTs were found to illustrate a characteristic pattern, which differs from that of the adjacent tissue. When the cursor is placed lateral to the TT, the appearance of the stratosphere sign31 (a sign that a pneumothorax is present) is evident, with no differentiation between the lung and the chest wall. When the cursor is placed over the TT, there is a characteristic appearance: absence of wave-forms below the level of the TT. We have termed this new finding “the black-out sign.” This appearance was noted for all tubes where M-mode was performed, regardless of TT size or type (Figures 4 and 5). If the cursor is placed over a rib, a similar appearance can be seen due to the absence of echoes beyond the rib. However, since the cursor is placed over the tube, which is seen beyond the pleural line, as compared to rib, which is above the pleural line, the confirmation of TT placement can be done using M-mode.
DISCUSSION
This study is the first of its kind to attempt to use US for TT evaluation on live human patients observing different transducer types and different techniques. Since TTs travel from superficial subcutaneous tissues through the pleural line into the pleural space, we chose to evaluate the TT by using both low- and high-frequency transducers. Many studies have illustrated that bedside US helps to decrease the risk of invasive procedures and improves patient care. If US can be used to aid in TT positioning by lending knowledge of malpositioning prior to breaking sterile field, it may also decrease patient morbidity from TT placement.
The current Advanced Trauma Life Support guidelines for the initial evaluation of TT placement is with CXR, followed by the gold standard CT.3 These modalities require radiation exposure. If tube repositioning is necessary, additional risks are incurred.18,30,32 Bedside CXRs have proven unreliable in the determination and evaluation of TT placement.11,32,33 CXR detects only 20% of malpositioned TT compared to CT.12,34 However, CTs take time, require mobilization of the patient and hemodynamic stability for patient transfer, and expose the patient to increasing doses of radiation.6,11 Therefore, another imaging modality is needed; US may be the perfect tool. US can be done quickly at the bedside, poses no risk to the patient, and does not expose the patient to radiation. US has been used to look at the placement of various other tubes including endotracheal tubes,9,10 nasogastric tubes,35 as well as nephrostomy stents and drains.36 However, this has not been studied for TT placement. This study is a pilot study and is the first of its kind, which illustrates that US can be used to visualize the TT and its placement in the pleural space. Its use will not prevent initial malpositioning but may lead to earlier detection of malpositioning (prior to breaking sterile field) if it is performed before a confirmatory CXR.
Our study was a pilot study that looked at a series of US images and clips taken from patients with TTs in place using both high- and low-frequency transducers to determine if US could evaluate TT position. Linear transducers have a greater spatial resolution and are, therefore, better able to distinguish between objects within the subcutaneous space. The phased-array transducers are able to view deeper objects but have decreased spatial resolution.19 In all 21 cases, we could easily visualize the TT as an anechoic circular structure with shadowing in its transverse view and an anechoic linear structure in its longitudinal view. However, in order to visualize the TT entering the pleural space for proper placement, it is important to see the TT in relation to the subcutaneous structures (Figure 2). Therefore, the linear transducer was subjectively determined to be optimal as it led to increased differentiation between tissues and structures. In our study, the research team agreed that the linear transducer more accurately illustrated the TT course through the subcutaneous tissue into the pleural space. As in a more recent study using cadavers, Salz et al37 visualized the TT in the subcutaneous tissue but also saw the TT disappear from visualization after it entered the intrathoracic region, which they also concluded suggests intrathoracic placement. However, our study is on emergency and surgical patients and also illustrates that the TT can be seen past the pleural line if fluid persists in the intrathoracic cavity in the region of TT placement. Since our study only looked at the entrance of the TT into the pleural space (not position within the chest cavity), the depth was not a large factor.
M-mode is normally used to evaluate objects in motion, such as the heart and lungs.17,19,31 However, when using M-mode in our study, we identified a distinct pattern. Unlike the expected finding of a stratosphere sign indicating pneumothorax, when the cursor is placed over the TT itself, we found a complete lack of waveform from the level of the TT and below. We termed this the black-out sign. It was found in 100% of the cases with both the phased array and linear transducers. The black-out sign was evident when the TT was subcutaneous and intrathoracic (Figures 4 and and5).5). Therefore, it helps TT identification.
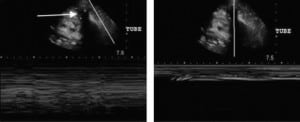
We found that the best technique for TT identification and positioning involves placing the linear transducer adjacent to the TT insertion site perpendicular to the tube, evaluating it in transverse orientation, eliciting the anechoic circular shadowing structure in 2-dimensional gray scale. The linear transducer should then be advanced along the pathway of the TT distal to the insertion site, visualizing the TT course through the subcutaneous tissue passing the hyperechoic pleural line and entering the pleural space. Lastly, for confirmation, M-mode should be used to elicit the characteristic black-out sign. The linear transducer can also be placed parallel to the TT, evaluating it in its longitudinal orientation, and advanced to visualize the TT entering the pleural space. Therefore, the TT can be viewed in 2 different planes to assure its positioning within the pleural space.
Age, gender, reason for TT placement, and tube contents (air vs serosanguineous) did not subjectively affect image acquisition or interpretation.
As the scope of practice widens and emergency physicians gain more confidence in the practice of US, the possibilities for US utilization grow. We have found that bedside US is able to show TT placement within the thoracic cavity and aid in differentiating the TT from the surrounding subcutaneous tissues. Noting significant limitations, this study is a proof-of-concept pilot study to show that bedside US can identify TT placement within the thoracic cavity.
LIMITATIONS
Several limitations exist in our study. This was a proof-of-concept pilot study, with a limited number of enrolled patients, decreasing the generalizability of the study. However, we believe that given the current paucity of information regarding US and TTs, our results are significant in that we are describing a new application for bedside US and a novel technique. In addition, although the sonographer was blinded to CXR and CT results, it is generally presumed that the TT was intrathoracic based on the fact that it had been left in place after chest radiographs and CTs were performed. By not studying TTs that were malpositioned, we were unable to compare the findings between TTs that are malpositioned and those that are properly positioned. However, it can be inferred from our findings that if the TT can be visualized, it likely is within subcutaneous tissues of the chest wall. However, if it disappears from view beyond the pleural line, it can be inferred that it is within the pleural space. Since only 15% of TTs are malpositioned within the subcutaneous tissues of the chest wall itself, this technique would not decrease the majority of malpositioned chest tubes, including those that are intraparenchymal and subdiaphragmatic. Also, since the primary investigator was present during the data collection and interpretation, it is difficult to generalize these skills to a typical ED sonographer. More studies will need to be done in the future to look at the generalizability of this study.
CONCLUSION
Bedside US may be able to determine TT position within the pleural space as well as distinguish the TT from surrounding subcutaneous tissue through a unique M-mode appearance termed the black-out sign. The optimal technique is to use the high-frequency linear transducer (subjectively found to be the best at differentiating between the subcutaneous structures) to visualize the TT in 2 planes, transverse and longitudinal, advance the transducer along the length of the TT, and watch it course through subcutaneous tissue, pass through the pleural line, and enter the pleural space. This can lead to further studies to determine (1) if this is applicable in real time, (2) if it will decrease time and cost in patient care, and (3) whether it will expedite transport of the patient to the operating room or ICU. On a larger scope, it could also then be used in austere situations, such as those encountered in disasters and in the wilderness, where higher levels of imaging are not available. Studies in larger populations and during real-time placement are needed to evaluate the further utility of this new technique. Thoracostomy tube evaluation is a very promising new application of bedside US.
Footnotes
Supervising Section Editor: Seric S. Cusick, MD
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Address for correspondence
Jamie A. Jenkins, MD
Stanford University /Kaiser Permanente Emergency Medicine Residency Program, Division of Emergency Medicine, Department of Surgery
Emergency Medicine Offices, 300 Pasteur Dr, Alway M121, Palo Alto, CA 94304
E-mail: jamiegoodis@gmail.com
REFERENCES
1. Fitzgerald M. Pleural decompression and drainage during trauma reception and resuscitation. Injury.2008;39:9–20. [PubMed]
2. Heng K, Bystrzycki A, Fitzgerald M, et al. Complications of intercostal catheter insertion using EMST techniques for chest trauma. ANZ J Surg. 2004;74:420–423. [PubMed]
3. Kool DR, Blickman JG. Advanced Trauma Life Support. ABCDE from a radiological point of view.Emerg Radiol. 2007;14:135–141. [PMC free article] [PubMed]
4. Rowan KR, Kirkpatrick AW, Lui D, et al. Traumatic pneumothorax detection with thoracic US: correlation with chest radiography and CT—initial experience. Radiology. 2002;225:210–214. [PubMed]
5. Karmy-Jones R, Holevar M, Sullivan RJ, et al. Residual hemothorax after chest tube placement correlates with increased risk of empyema following traumatic injury. Can Respir J. 2008;15:255–258.[PMC free article] [PubMed]
6. Baldt MM, Bankier AA, Germann PS, et al. Complications after emergency tube thoracostomy: assessment with CT. Radiology. 1995;19:539–543. [PubMed]
7. Bouhemad B, Zhang M, Lu Q, et al. Clinical review: bedside lung ultrasound in critical care practice.Crit Care. 2007;11:205. [PMC free article] [PubMed]
8. Osinowo O, Softah AL. Eid Zahraini M. Ectopic chest tube insertions: diagnosis and strategies for prevention. Afr J Med Med Sci. 2002;31:68–70.
9. Chun R, Kirkpatrick AW, Sirois M, et al. Where’s the tube? Evaluation of hand-held ultrasound in confirming endotracheal tube placement. Prehosp Disaster Med. 2004;19:366–369. [PubMed]
10. Sustic A. Role of ultrasound in the airway management of critically ill patients. Crit Care.2007;35:173–177.
11. Mandavia DP, Joseph A. Bedside echocardiography in chest trauma. Emerg Med Clin North Am.2004;22:601–619. [PubMed]
12. Wright J, Jarman R, Connolly J, et al. Echocardiography in the emergency department. Emerg Med J. 2009;26:82–86. [PubMed]
13. Salen P, Melanson S, Buro D. ED screening to identify abdominal aortic aneurysms in asymptomatic geriatric patients. Am J Emerg Med. 2003;21:133–135. [PubMed]
14. Kendall JL, Shimp RJ. Performance and interpretation of focused right upper quadrant ultrasound by emergency physicians. J Emerg Med. 2001;21:7–13. [PubMed]
15. Bahner D, Blaivas M, Cohen HL, et al. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2008;27:313–318.[PubMed]
16. Mock C, Lormand JD, Goosen J, et al. In: Guidelines for Essential Trauma Care. Geneva, Switzerland: World Health Organization; 2004. Management of abdominal injury; pp. 34–36.
17. Aylwin C, Brohi K, Davies GD, et al. Prehospital and in-hospital thoracostomy: indications and complications. Ann R Coll Surg Engl. 2008;90:54–57. [PMC free article] [PubMed]
18. Heller M, Melanson S. Ultrasound in emergency medicine. Am J Emerg Med. 1996;14:235.[PubMed]
19. Jones R. Handbook of Ultrasound in Early Pregnancy. Canton, OH: Haines International;; 2006. Instrumentation and knobology; pp. 6–25. In.
20. Dulchavsky SA, Hamilton DR, Diebel LN, et al. Thoracic ultrasound diagnosis of pneumothorax. J Trauma. 1999;47:970–971. [PubMed]
21. Legome E, Pancu D. Future applications for emergency ultrasound. Emerg Med Clin North Am.2004;22:817–827. [PubMed]
22. Targhetta R, Bourgeois JM, Chavagneux R, et al. Diagnosis of pneumothorax by ultrasound immediately after ultrasonically guided aspiration biopsy. Chest. 1992;101:855–856. [PubMed]
23. Lichtenstein D, Meziere G, Biderman P, et al. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25:383–388. [PubMed]
24. Lichtenstein D, Meziere G, Biderman P, et al. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26:1434–1440. [PubMed]
25. Chan SS. Emergency bedside ultrasound to detect pneumothorax. Acad Emerg Med. 2003;10:91–94. [PubMed]
26. Stone MB, Secko MA. Bedside ultrasound diagnosis of pulmonary contusion. Pediatr Emerg Care.2009;25:854–855. [PubMed]
27. Platz E, Cydulka R, Werner S, et al. The effect of pulmonary contusions of lung sliding during bedside ultrasound. Am J Emerg Med. 2009;27:363–365. [PubMed]
28. Chan SS. Emergency bedside ultrasound for the diagnosis of rib fractures. Am J Emerg Med.2009;27:617–620. [PubMed]
29. Wright J, Jarman R, Connolly J, et al. Echocardiography in the emergency department. Emerg Med J. 2009;26:82–86. [PubMed]
30. McEwan K, Thompson P. Ultrasound to detect haemothorax after chest injury. Emerg Med J.2007;24:581–582. [PMC free article] [PubMed]
31. Lichtenstein DA, Mezière G, Lascola N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33:1231–1238. [PubMed]
32. Gayer G, Rozenman J, Hoffman C, et al. CT diagnosis of malpositioned chest tubes. Br J Radiol.2000;73:786–790. [PubMed]
33. Remerand F, Luce V, Badachi Y, et al. Incidence of chest tube malposition in the critically ill: a prospective computed tomography study. Anesthesiology. 2007;106:1112–1119. [PubMed]
34. Lima KE, Tai SC, Chan CY, et al. Diagnosis of malpositioned chest tubes after emergency tube thoracostomy. Is computed tomography more accurate than chest radiograph? Clin Imaging.2005;29:401–405.
35. Hernandez-Socorro CR, Marin J, Ruiz-Santana S, et al. Bedside sonographic guided versus blind nasoenteric feeding tube placement in critically ill patients. Crit Care Med. 1996;24:1690–1694.[PubMed]
36. Cosgrove DO, Chan KE. Renal transplants: what ultrasound can and cannot do. Ultrasound Q.2008;24(2):77–87. [PubMed]
37. Salz TO, Wilson SR, Liebmann O, et al. An initial description of a sonographic sign that verifies intrathoracic chest tube placement. Am J Emerg Med. 2010;28:626–630. [PubMed]


