| Author | Affiliation |
|---|---|
| Paul Walsh, MB, BCh, BAO, MSc | Kern Medical Center, Department of Emergency Medicine and David Geffen School of Medicine, University of California Los Angeles |
| Christina Overmeyer, BS | Research and Development Department, Medical Diagnostic Laboratories, Hamilton NJ |
| Lauren Kimmel, BS | Research and Development Department, Medical Diagnostic Laboratories, Hamilton NJ |
| Melanie Feola, BS | Research and Development Department, Medical Diagnostic Laboratories, Hamilton NJ |
| James Pusavat, BS | Kern Medical Center, Department of Pathology |
| Tuan Anh Nguyen | Kern Medical Center, Department of Emergency Medicine and David Geffen School of Medicine, University of California Los Angeles |
| Sam Kuan, MB, BCh, BAO | Kern Medical Center, Department of Emergency Medicine and David Geffen School of Medicine, University of California Los Angeles |
| Kirt Emery, MPH | Kern County Department of Public Health |
| Martin Rosengreen, MD | Kern Medical Center, Department of Emergency Medicine and David Geffen School of Medicine, University of California Los Angeles |
| Eli Mordechai, PhD | Research and Development Department, Medical Diagnostic Laboratories, Hamilton NJ |
| Martin E. Adelson, PhD | Research and Development Department, Medical Diagnostic Laboratories, Hamilton NJ |
ABSTRACT
Introduction:
The clinical presentation of Bordetella pertussis can overlap with that of respiratory syncytial virus (RSV); however, management differs. First, the prevalence of B. pertussis is less than 2% among patients screened for RSV, and second the prevalence of B. parapertussis is also less than 2% among these patients.
Methods:
Nasal washings submitted to a clinical laboratory for RSV screening were tested for B. pertussis and B. parapertussis, using species-specific real-time polymerase chain reaction (PCR) assays. These were optimized to target conserved regions within a complement gene and the CarB gene, respectively. A Bordetella spp. genus-specific real-time PCR assay was designed to detect the Bhur gene of B. pertussis, B. parapertussis, and B. bronchiseptica. RSV A and B subtypes were tested by reverse transcription-PCR.
Results:
Four hundred and eighty-nine clinical samples were tested. There was insufficient material to complete testing for one B. pertussis, 10 RSV subtype A, and four RSV subtype B assays. Bordetella pertussis was detected in 3/488 (0.6%) (95% CI 0.1% to 1.8%), whileB. parapertussis was detected in 5/489 (1.0%) (95% CI 0.3% to 2.4%). Dual infection of B. pertussis with RSV and of B. parapertussis with RSV occurred in two and in three cases respectively. RSV was detected by PCR in 127 (26.5%).
Conclusion:
The prevalence of B. pertussis in nasal washings submitted for RSV screening was less than 2%. The prevalence of parapertussis may be higher than 2%. RSV with B. pertussisand RSV with B. parapertussis coinfection do occur.
INTRODUCTION
The clinical presentation of Bordetella pertussis can overlap with and be clinically indistinguishable from that of respiratory syncytial virus (RSV).1,2 The potential for morbidity and mortality is higher with B. pertussis.2,3 Apnea occurs in 15.9% of previously healthy infants less than six months of age who contract pertussis.4 Apnea rates for RSV are lower, even in hospitalized infants.5 This risk is largely confined to infants less than two months of age and to those with co-morbidities.6–9 Admission criteria reflect these differences with mandatory admission recommended for those with pertussis up to six months of age.10 Mandatory admission for infants with RSV is controversial, with conflicting published evidence and opinions, but many opt to admit all less than two months of age.11,12,13
RSV epidemiological trends have remained steady14 whereas pertussis infections have climbed both locally (Kern County Department of Public Health data) and nationally15with genomic subtypes not contained in vaccinations being increasingly represented.16Previous work among children admitted to intensive care for bronchiolitis in the UK, and among infants less than six months of age hospitalized with bronchiolitis in Finland, suggests that despite the apparently viral nature of their illness (typically RSV), pertussis is under diagnosed.1,17 However this experience is not universal with some reporting the opposite.18 These studies raise the question whether infants and toddlers screened for RSV should also be screened for B. pertussis.
While baseline population prevalence data can help inform such a decision, these estimates from public health departments are of limited value. They reflect cases with more typical presentations in which the diagnosis was clinically suspected; even then they are prone to under reporting.19,20 Determining the overall baseline-expected prevalence of B. pertussis in patients with presentations more typically associated with RSV requires large numbers. It also requires a sample representative of the full spectrum of RSV disease that presents for treatment (primarily bronchiolitis). We tested de-identified specimens previously submitted for RSV screening to determine the prevalence of B. pertussis in this population.
We considered that a prevalence of less than 2% would represent an acceptable miss rate for most emergency physicians (EPs) for pertussis and tested the hypothesis that the prevalence of B. pertussis is less than 2% in nasal washings screened for RSV. Our secondary hypothesis was that the prevalence of B. parapertussis is less than 2% in nasal washings screened for RSV.
METHODS
Our institutional review board exempted the study from review.
Setting
A single clinical laboratory serving an emergency department (ED), pediatric clinic, family practice clinic and a county hospital and a diagnostic testing laboratory specializing in the use of molecular amplification assays for the detection of viruses, bacteria, and fungi in biological specimens.
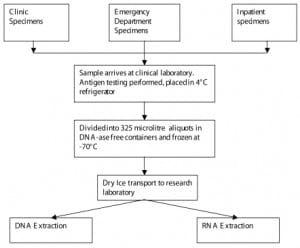
Nasal washings samples collected between October 2005 and May 2006 were first tested for the RSV antigen by the clinical laboratory. They were collected in test tubes without additives using normal saline. The remaining part of each sample was refrigerated to 4°C prior to being divided into 325 μl aliquots into DNA- ase-free transport containers and frozen to −70°C. The flow of specimens is shown in Figure 1.
Upon receipt at the diagnostic testing laboratory the specimens were thawed, and DNA was extracted using an automated Corbett Robotics X-tractor Gene system. Extracted DNA from these specimens were subsequently tested by Bordetella pertussis and Bordetella parapertussis species-specific real-time polymerase chain reaction (PCR) assays designed and optimized to target conserved regions within a complement gene and the CarB gene, respectively. A Bordetella spp. genus-specific real-time PCR assay was also designed to detect the Bhur gene of B. pertussis, B. parapertussis, and B. bronchiseptica. The characteristics and detection limits of this PCR assay (detection limit for B. pertussis 100 copies per reaction, detection limits for B. parapertussis 10 copies per reaction) are shown in Figures 2 and and3.3. Specificity was assessed by testing the assay against known samples of the viral, bacterial, and fungal pathogens listed in Table 1. No amplification for any of these agents was detectable for the B. pertussis, B. parapertussis or Bordetella ssp. assays. These assays are available for clinical use from a commercial CLIA approved laboratory.
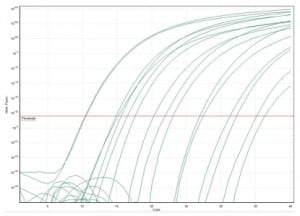
B. pertussis range of detection and standard curve ranging from 1 × 1010 copies to 1 × 102 copies.
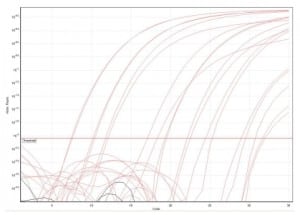
B. parapertussis range of detection and standard curve ranging from 1 × 1010 copies to 1 × 102 copies.
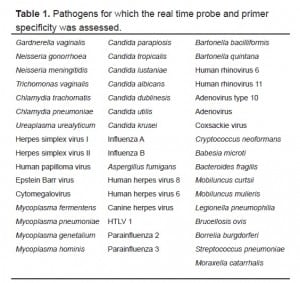
Pathogens for which the real time probe and primer specificity was assessed.
For the RSV assays, RNA was extracted from the washings using either a modified protocol with the Corbett Robotics X-tractor Gene System or with the QIAamp Viral RNA purification kit (Qiagen, Valencia, CA). Extracted RNA was subsequently tested for RSV-A and RSV-B using two separate one-step reverse transcription PCR assays targeting genes, which encodes a phosphoprotein for each strain. The detection threshold for RSV-A and RSV-B was 100 copies per reaction.
Because we were not allowed to use patient identifiers, we used total population characteristics for all RSV samples submitted to the clinical laboratory during the study period to estimate population characteristics.
Statistical Analysis
Prevalence and exact confidence intervals were calculated for B. pertussis, B. parapertussis and RSV and co-infection. We tested the hypotheses that the proportion of cases screening positive for B. pertussis and B. parapertussis would exceed 2% with exact methods using STATA 9.2 (Stata Corp LP, College Station, TX).
RESULTS
The median age of the population sampled was 4.8 months (IQR 8.2); 356/648 (54.9%) were male. Six hundred and forty-eight specimens were received at the clinical lab. Most of these, 571 (88.4%) came from the ED, 49 (7.6%) from the pediatric and family practice clinics, and 26 (4.0%) from inpatients. Four hundred and eighty-nine (75.5%) of these submitted clinical samples were captured for additional testing. There was insufficient material to complete testing for one B. pertussis, 10 RSV subtype A, and three RSV subtype B assays. Bordetella pertussis was detected in 3/488 (0.61%) (95% CI 0.13% to 1.79%) while B. parapertussis was detected in 5/489 (1.02%) (95% CI 0.33% to 2.37%). Dual infection with B. pertussis and RSV was found in two cases. Dual infection with B. parapertussis and RSV occurred in three cases. The results are detailed in Table 2. One case of B. bronchiseptica (‘kennel cough’) was also found. RSV A was found in 75/479 (16%) samples and RSV B in 52/486 (11%). Local prevalence trends for the preceding 10 years are shown in Figure 4 to place the results in context.
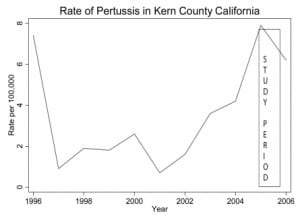
The context in which our sampling was performed. The box represents the sampling period.
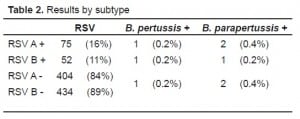
Results by subtype
DISCUSSION
Despite the rising local and national trend of reported cases of B. pertussis at the time of the study, we found very low rates of co-infection of B. pertussis and RSV. Our study does provide some evidence for clinicians who discount concerns about missing pertussis during RSV season, even during the current apparent resurgence of pertussis.
Our results are consistent with that of a retrospective study of 166 children less than six-years-old hospitalized with lower respiratory tract symptoms in which a 0.6% (95% CI: 0.0% to 3.3%) prevalence was noted.1 However, Siberry et al.18 was performed at a low point in local disease prevalence (five cases in all age groups in Baltimore) and drawn from an older population than ours; the studies are therefore not directly comparable. Our results contrast with those of Korppi and Hiltunin,17 which found an 8% prevalence in infants hospitalized with bronchiolitis who were less than six months old.
Our methodology allowed us to obtain large numbers of clinical specimens but not to obtain individual level clinical information on the patients from whom they were obtained, much less the clinicians’ reasons for ordering the test. This is a feature of studies using so called “discarded clinical specimens.” Such studies’ results are most useful inferentially when they find a high or a low prevalence of an infectious agent. They are useful in triangulation, i.e. supporting a particular hypothesis by three different research methodologies. Triangulation is warranted when investigating conditions with high morbidity (such as causes of apnea) or when existing research provides conflicting answers.
This study suggests that when the community prevalence of pertussis is low, (our reported prevalence was 7.9/100,000 during the study period), and a 2% miss rate is acceptable to clinicians, routine screening for pertussis in patients suspected of having RSV is unwarranted. This is in contrast to studies that have looked at selected subgroups and found B. pertussis to be common. The definitiveness of this statement will depend on the results being replicated over a number of seasons.
LIMITATIONS
Local test-ordering patterns may help explain our low RSV rate. The sampling would have included patients screened for RSV early and late in the bronchiolitis season when other viruses are generally responsible. In-patient screening samples required for cohorting would also have been included in this analysis. RSV testing may have been obtained in cases of fever without source, or as part of apparent-life threatening event (ALTE) evaluations. False-negative PCR results for RSV are also a likely potential reason. This may in part be due to dilution of virus inherent in the collection of nasal aspirates. Initial specimen collection used unpreserved saline in clinical test tubes. RNA is less stable than DNA. During the uncontrolled phase (specimen going from patient to clinical laboratory) and despite refrigeration prior to aliquoting into DNA-ase-free containers, some RNA degradation could have occurred. Nasal aspirates have been shown to be markedly inferior to universal transport medium for room temperature (UTM-RT) based collection methods for PCR-based testing for respiratory viruses in general and RSV in particular.21,22 While this may artificially lower our detection of RSV it is less likely to have such an effect on Bordetella spp. Detection of bacterial DNA is much less susceptible to the collection method used (unpublished data). All of these factors may account for the difference between RSV detection in this and another contemporaneous study in our institution where a clinical diagnosis of bronchiolitis was the entry criterion and in which RSV prevalence was 55%.23
The use of nasal washings rather than nasopharyngeal swabs (NPS) could potentially have decreased the yield of pertussis. While only NPS are considered acceptable for culture, the sensitivity of PCR mitigates against the potential loss of sensitivity by our methods.
Mistaken attribution of respiratory illness to B. pertussis has been described due to poor PCR assay specificity but high sensitivity.24 We feel this is unlikely to be the case in our study based on the specificity testing results for our assay and the actual study results obtained.
We have not addressed here the broader epidemiology of those clinical presentations that typically prompt RSV testing. The clinical role for testing for these other agents is currently evolving. We could not control the age of patients whose samples were included; however, based on the age profile of the patients whose samples were submitted, our results should be considered applicable only to infants and toddlers.
We included only samples from a single clinical laboratory serving a single county system of clinics and hospital sites. Although this system serves a mixed urban, suburban and rural population, our patients tend to be drawn from lower socio-economic groups. This would tend to increase the prevalence of infectious diseases in general and may overestimate the prevalence that would be found in a more affluent setting. On the other hand vaccine refusal, which increases vulnerability to pertussis, tends to occur in higher socio-economic groups. Local DPT vaccination and pertussis patterns mirror those of the rest of the state and country as a whole.
Our results must be viewed in the context of the overall prevalence of pertussis within the community. A higher prevalence could have changed our results, although the degree of change necessary to change the outcome is speculative. Nonetheless, although low by historical standards, prevalence was at a 10-year high during our study period. This is based on Department of Public Health reported case, data that inevitably carry their own limitations. Although the number of cases reported is low, it is likely an underestimate. It is estimated that between one-third and one in 20 of actual pertussis cases are reported.19,20 Nonetheless, the increasing trend of reported cases matches that for state and national reporting.
Our geographic locale is large. It is also a relatively sparsely populated, poor, and a medically underserved county with very low per person medical care expenditure,25again suggesting that the actual number of pertussis cases is probably higher than that reported.
Future reports will address these limitations by enrolling only ED patients and addressing clinical variables and outcomes over a number of seasons.
CONCLUSION
In a combined outpatient clinic, ED, and inpatient population, the prevalence of B. pertussis in nasal washings submitted for RSV antigen screening was less than 2%. The prevalence of parapertussis may have been higher than 2%. RSV with B. pertussis and RSV with B. parapertussis coinfection did occur.
Footnotes
Supervising Section Editor: Robert W. Derlet, MD
Submission history: Submitted November 12, 2007; Revision Received April 18, 2008; Accepted May 15, 2008.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Paul Walsh, MB, BCh, BAO, MSc, Department of Emergency Medicine, Kern Medical Center, Bakersfield, CA and David Geffen School of Medicine, University of California Los Angeles
Email yousentwhohome@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Crowcroft NS, Booy R, Harrison T, et al. Severe and unrecognised: pertussis in UK infants. Arch Dis Child. 2003;88:802–806. [PMC free article] [PubMed]
2. Sotomayor J, Weiner LB, McMillan JA. Inaccurate diagnosis in infants with pertussis. An eight-year experience. Am J Dis Child. 1985;139:724–727. [PubMed]
3. Smith C, Vyas H. Early infantile pertussis; increasingly prevalent and potentially fatal. Eur J Pediatr. 2000;159:898–900. [PubMed]
4. Heininger U, Klich K, Stehr K, Cherry JD. Clinical Findings in Bordetella pertussis Infections: Results of a Prospective Multicenter Surveillance Study. Pediatrics.1997;100:e10. [PubMed]
5. Church NR, Anas NG, Hall CB, Brooks JG. Respiratory syncytial virus-related apnea in infants. Demographics and outcome. Am J Dis Child. 1984;138:247–250. [PubMed]
6. Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr.1995;126:212–219. [PubMed]
7. Foo AL, Chay OM, Hiew J, Tan CK, Lim KW, Cheng HK. Severe bronchiolitis in children.J Singapore Paediatr Soc. 1991;33:165–168. [PubMed]
8. Van Steensel-Moll HA, Van dV, Bos AP, Rothbarth PH, Neijens HJ. Respiratory syncytial virus infections in children admitted to the intensive care unit. Pediatrie.1989;44:583–588. [PubMed]
9. Neilson KA, Yunis EJ. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol. 1990;10:491–502. [PubMed]
10. Fleischer G. Infectious Disease Emergencies. In: Fleischer G, Ludwig S, editors.Textbook of pediatric emergency medicine. 4. Philadelphia: Lippincott Williams; 1999. pp. 725–93.
11. Ruddy R, Gittleman M. Pedatric Emergencies. Atlanta: American Health Consultants; 2001. Pediatric Emergencies; pp. 135–50.
12. Willwerth BM, Harper MB, Greenes DS. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med. 2006;48:441–447.[PubMed]
13. Arms JL, Ortega HW, Finkelstein M, Reid SR. Apnea complicating RSV infection in infancy. Ann Emerg Med. 2006;48:95.
14. Respiratory and Enteric Virus National Trends. [Accessed October 20, 2007];Centers for Disease Control. 2005 Available at:http://www.cdc.gov/ncidod/dvrd/revb/nrevss/trends.htm.
15. Cherry JD. The Epidemiology of Pertussis: A Comparison of the Epidemiology of the Disease Pertussis With the Epidemiology of Bordetella pertussis Infection. Pediatrics.2005;115:1422–1427. [PubMed]
16. Hardwick TH, Cassiday P, Wayent R, Bisgard K, Sanden G. Changes in Predominance and Diversity of Genomic Subtypes of Bordetella pertussis Isolated in the United States, 1935–1999. Emerging Infectious diseases. 2002;8:44–49. [PMC free article] [PubMed]
17. Korppi M, Hiltunen J. Pertussis is common in nonvaccinated infants hospitalized for respiratory syncytial virus infection. Pediatr Infect Dis J. 2007;26:316–318. [PubMed]
18. Siberry GK, Paquette NR, Ross TL, Perl TM, Valsamakis A. Low Prevalence of Pertussis Among Children Admitted With Respiratory Symptoms During Respiratory Syncytial Virus Season. Infect Control Hosp Epidemiol. 2006;27:95–97. [PubMed]
19. Sutter RW, Cochi SL. Pertussis hospitalizations and mortality in the United States 1985–1988. Evaluation of the completeness of national reporting. JAMA. 1992;267:386–391. [PubMed]
20. Ward JL, Cherry JD, Chang SJ. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med. 2005;353:1555–1563. [PubMed]
21. Walsh P, Overmeyer C, Pham K, Michaelson S, Pusavat J, Tran T, Mordechai E, Adelson M. Comparison of RSV-A RNA Recovery by PCR Using Different Methods of Specimen Preservation. Annals of emergency medicine. 2007;50(3):S38.
22. Walsh P, Lim-Overmeyer C, Gofman L, et al. Pediatric Respiratory Infectious Disease Analysis: UTM-RT versus Flocked Swab Nasal Collections. Western Journal of Emergency Medicine. 2008;9:72.
23. Walsh P, Caldwell J, McQuillan KK, Friese S, Robbins D, Rothenberg SJ. Comparison of nebulized epinephrine to albuterol in bronchiolitis. Acad Emerg Med. 2008;15:305–313.[PMC free article] [PubMed]
24. Centers for Disease Control and Prevention. Outbreaks of Respiratory Illness Mistakenly Attributed to Pertussis — New Hampshire, Massachusetts, and Tennessee, 2004–2006. MMWR. 2007;56:837–842. [PubMed]
25. Starr G. Comparison of funding to county hospitals. Blue Ribbon Task Force Recommendations. (Kern Medical Center). February 26, 1997. Kern County Board of Supervisors, Blue Ribbon Task Force.