| Author | Affiliation |
|---|---|
| Erin M. Fletcher | San Diego State University, San Diego, California University of California, San Diego, Department of Emergency Medicine, San Diego, California Rady Children’s Hospital, San Diego, California |
| Ghazala Sharieff, MD, MBA | University of California, San Diego, Department of Emergency Medicine, San Diego, California Rady Children’s Hospital, San Diego, California University of California, San Diego, Department of Pediatrics, San Diego, California |
Introduction Methods Results Discussion Conclusion
ABSTRACT
Introduction: This study aims to characterize the population of patients presenting to a pediatric emergency department (ED) for a first complex febrile seizure, and subsequently assess the rate of acute bacterial meningitis (ABM) occurrence in this population. Furthermore, this study seeks to identify whether a specific subset of patients may be at lesser risk for ABM or other serious neurological disease.
Methods: This retrospective cohort study reviewed the charts of patients between the ages of 6 months to 5 years of age admitted to an ED between 2005 and 2010 for a first complex febrile seizure (CFS). The health information department generated a patient list based on admission and discharge diagnoses, which was screened for patient eligibility. Exclusion criteria included history of a complex febrile seizure, history of an afebrile seizure, trauma, or severe underlying neurological disorder. Data extracted included age, gender, relevant medical history, descriptions of seizure, treatment received, and follow-up data. Patients presenting with two short febrile seizures within 24 hours were then analyzed separately to assess health outcomes in this population.
Results: There were 193 patients were eligible. Lumbar puncture was performed on 136 subjects; it was significantly more likely to be performed on patients that presented with seizure focality, status epilepticus, or a need for intubation. Fourteen patients were found to have pleocytosis following white blood cell count correction, and 1 was diagnosed with ABM (0.5% [95% confidence interval: 0.0–1.5, n=193]). Forty-three patients had 2 brief febrile seizures within 24 hours. Of the 43, 17 received lumbar puncture while in the ED. None of these patients were found to have ABM or other serious neurological disease.
Conclusion: ABM is rare in patients presenting with a first complex febrile seizure. Patients presenting only with 2 short febrile seizures within 24 hours may be less likely to have ABM, and may not require lumbar puncture without other clinical symptoms of neurological disease.
INTRODUCTION
Febrile seizures are seizures that occur in conjunction with high fever. They occur in up to 5% of children, typically between the ages of 6 months and 5 years.1–2 Febrile seizures are classified as simple or complex. Simple febrile seizures are generalized, last less than 15 minutes, and do not recur within 24 hours. All others are considered complex and comprise 35% of first time febrile seizures.3 Most children do not experience long-term effects due to simple febrile seizures.4 However, complex febrile seizures may have been suggested to increase the risk of epilepsy in some children, particularly those with previously existing neurological abnormalities.2
A practice parameter developed by the American Academy of Pediatrics (AAP) for the treatment of children presenting with a simple febrile seizure (SFS) provides a guideline for treatment. The SFS practice parameter focuses on identifying the source of fever rather than performing a standard seizure work up, and examining for signs of encephalitis or meningitis by performing a lumbar puncture on children presenting with clinical signs of neurological disease.4 No such guideline exists for children presenting with a first complex febrile seizure, and current treatment plans for this population vary greatly among pediatric emergency providers.1,5 Medical providers often elect to perform lumbar punctures on these patients to rule out acute bacterial meningitis (ABM), although recent literature has shown that ABM is rarely diagnosed in the absence of other clinical signs and symptoms of serious neurological disease.6–8
Our study primarily aimed to characterize the pediatric population presenting with a first complex febrile seizure and determine the likelihood of ABM in these patients. Furthermore, recent literature has suggested that lumbar puncture may not be beneficial for patients presenting with two brief febrile seizures within a 24-hour period.6 Our goal was to determine whether this specific subpopulation of patients is at a lesser risk of ABM or serious neurological disease.
METHODS
Study Design
This study was a retrospective cohort review of patients admitted to an urban pediatric emergency department (ED), which sees approximately 71,000 patients annually. This study was approved by the University of California, San Diego Institutional Review Board and the Rady Children’s Hospital Research Administration office.
Study Setting and Population
This study retrospectively reviewed electronically available physician notes for patients who met study criteria. Inclusion criteria were being seen in the ED between January 1, 2005, and September 1, 2010, for availability of electronic physician notes; ED diagnosis of complex febrile seizure or status epilepticus; and 6 months to 5 years of age at the time of ED visit. We chose this age group in order to be consistent with the classic age range of a simple febrile seizure. All patients presented to the ED within 24 hours of the seizure, and had one or more features of a complex seizure. Patients were excluded if they had a previous history of afebrile seizures; did not meet criteria for complex febrile seizures; had a preceding history of trauma; or had a history of significant neurological abnormalities or a history of trauma.
Study Protocol
Patients were identified by the hospital’s health information department, whichelectronically generated a list of patients seen in the ED with an assigned International Classification of Diseases (ICD)-9/10 code of either 780.32 for complex febrile convulsions or 345.3 for status epilepticus. This search included both admission and discharge diagnoses from the ED. It is unlikely to have excluded patients with a recorded diagnosis of bacterial meningitis rather than complex febrile seizure due to our institution’s billing practices, which include a full listing of all diagnoses. This list was manually screened by both authors to ensure that the inclusion and exclusion criteria were met.
Data Extraction
Charts by the attending physician, nurses, and emergency transport staff, as applicable, were reviewed for each patient by both authors. However, as our research objective remains simply to characterize this patient population and compare to existing literature on this topic, we assert no investigator bias. Attending physician notes were surveyed for physical examination data, specifically the presence of macrocephaly, Todd’s paralysis, petechiae, a prolonged postictal period, or a need for intubation. Each patient’s record was screened for hospital notes, if admitted, and any subsequent follow up at our institution within 7 days of discharge.
Definitions
Cerebrospinal fluid (CSF) pleocytosis was defined as CSF white blood cell (WBC) count of > 7/μL.6 In the case of blood-contaminated CSF samples, the authors used the correction equation: corrected CSF WBC count = (CSF WBC count – [CSF red blood cell count/500]).6 Acute bacterial meningitis was defined as either bacterial growth from a CSF sample taken within a week of the ED visit, or CSF pleocytosis with bacterial growth from a blood culture within a week of the ED visit.6 In the case of an uncertain ABM diagnosis, discharge diagnosis by the treating attending physician was considered final. Status epilepticus was defined as seizure activity without cessation for at least 30 minutes or recurrent seizures without regaining consciousness.9
Data Analysis
We performed data analysis using MYSTAT 12 (Chicago, Illinois), and calculated percentages and confidence intervals (CIs) using the Descriptive Statistics function. After characterization of the general population, we then analyzed separately patients presenting with 2 short febrile seizures within 24 hours to assess health outcomes in this population.
RESULTS
Case Identification
The subject list generated by the health information department listed 506 eligible patients; of those, 370 were between the ages of 6 months and 5 years of age. After screening for history of neurological abnormalities, afebrile seizure, complex febrile seizure, trauma, 193 eligible patients remained.
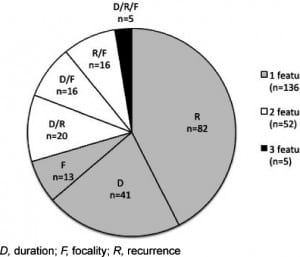
Background information on eligible patients is outlined in Table 1. The majority of the sample (70.5%) had a febrile seizure with only 1 complex feature, 26.9% demonstrated 2 complex features, and 2.6% exhibited all 3 complex features. Figure 1 shows the further breakdown of complex seizure features. More than half (54.4%) of the population received medication for seizure cessation. The median highest recorded temperature was 39.3°C (interquartile range [IQR]: 38.5–39.9).

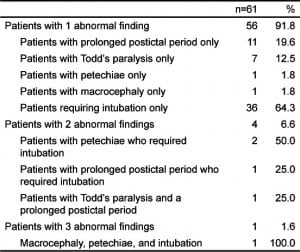
Upon physical exam, 132 patients (68.4%) had no notable manifestations of serious neurological disease; however, 40 patients required intubation in the ED. It can be difficult to determine whether the need for airway protection was a result of the natural course of the seizure, or an effect of the administration of anticonvulsants. We therefore recorded intubation as occurring before (n=5) or after (n=35) the administration of anticonvulsants to account for this uncertainty. The rest of these notable findings are outlined in Table 2.
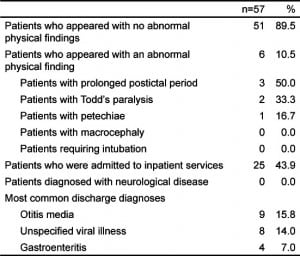
The most common discharge diagnoses for patients seen for a first complex febrile seizure were febrile illness (n=33), viral syndrome (n=26), and otitis media (n=24). Treating physicians admitted 139 patients; 131 were admitted to the hospital’s inpatient services, and 8 were transferred to other institutions due to insurance requirements. Follow-up data were available for 170 patients (86%), and included inpatient discharge summary; imaging studies (computed tomography, electroencephalogram, or magnetic resonance imaging); or follow-up visit to our hospital. As the admitting facility for our region, we are aware of patients admitted in our area, and it is unlikely that any children were re-admitted without our awareness.
Lumbar Puncture Administration
Lumbar puncture was performed on 70.5% of subjects in our ED. Factors that were associated with lumbar puncture administration were no prior history of simple febrile seizure (75.6%), seizure focality (84.0%), status epilepticus (91.3%), and requiring intubation (100.0%). Fifty-seven patients did not receive a lumbar puncture. None of these patients returned to this hospital with a diagnosis of ABM. Further description of the 57 patients who did not receive lumbar puncture can be found in Table 3.
CSF Results
The median corrected CSF WBC count was 1/μL (IQR: 0–3). Fifteen subjects had CSF pleocytosis; 8 required the CSF WBC correction formula for blood-contaminated CSF samples. Fourteen subjects (7.3%) had pleocytosis after WBC count correction (95% CI: 4.1–10.1), 7 of whom were diagnosed at discharge by the attending physician with a serious neurological disease. Figure 2 shows the health outcomes of these subjects; only one was found to have ABM.
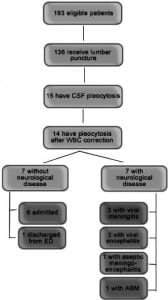
One 3-year-old male patient presented to our facility after being previously seen in a different ED for a simple febrile seizure. He had a total of 4 febrile seizures, one of which lasted 30 minutes. He was intubated after receiving anticonvulsants, but had no other physical indicator of neurological disease. After receiving a lumbar puncture, he was found to have CSF pleocytosis (WBC=12). He lacked CSF or blood culture growth, but antibodies for a mycoplasma bacterial species were identified in the blood. The attending physician assigned this patient a discharge diagnosis of bacterial meningitis, for which he was treated during a month-long inpatient stay. Based on our review, we conclude that the rate of ABM in our overall patient population (n=193) is 0.5% [95% CI: 0.0%–1.5%]).
Of the 7 patients with CSF pleocytosis and serious neurological disease all were admitted to the hospital for treatment and observation; 1 was transferred to another facility for insurance purposes. One returned to the ED after hospital discharge for follow-up during recovery from viral meningitis; he was found to be stable and was discharged home.
Sub-analysis of Patients with 2 Brief Febrile Seizures
Of the 193 patients included in this study, 43 were identified to present with only 1 seizures, without any report of focality or prolonged seizure duration. Of these 43, 7 experienced their second seizure in the ED and subsequently received anticonvulsants. Twenty patients in this sub-population had already been seen in an ED for their initial febrile seizure, and were discharged home with a diagnosis of simple febrile seizure. Only 1 of the 20 received a lumbar puncture at the initial visit due to the presence of petechiae, but her CSF appeared normal and no other physical signs of disease were apparent upon presentation. She and 8 other patients were discharged from their initial visit with antibiotics. Background data on these patients is summarized in Table 4.

Lumbar puncture was performed on 17 of these patients, not including the patient who received LP during her initial ED visit. One subject was found to have pleocytosis (WBC=11), but lacked growth of any organism from CSF or blood cultures and had no other physical manifestations of neurological disease. None of the patients who underwent a lumbar puncture had any growth from a CSF culture. Blood culture was performed on 22 patients, and no organism was isolated from any of these samples. None was assigned an admission or discharge diagnosis of bacterial meningitis, or other serious neurological disease.
During the course of treatment in our ED for complex febrile seizure, 10 patients required supplemental oxygen, but 0/43 required intubation. None of the patients presented with Todd’s paralysis or macrocephaly; however, 3 appeared to have a prolonged postictal period, and another previously mentioned patient presented with petechiae. Twenty patients were admitted for observation, including 2 by parental request. None of the patients had an additional seizure or any recorded adverse event during the course of inpatient stay. As in the patients who did not receive lumbar puncture, the 3 most common discharge diagnoses were unspecified viral illness (n=7), otitis media (n=6), and gastroenteritis (n=4); no child was diagnosed with a serious neurological disease. One patient returned after discharge due to complaints of herpangina. Follow-up data were available for all of the patients. None of the patients returned with reports of additional seizure or other neurological sequelae.
DISCUSSION
The importance of diagnosing acute bacterial meningitis in young pediatric patients is paramount; the disease progresses quickly and can cause long-term damage less than a day after symptoms arise.8Immediate medical attention is vital to the patient’s survival and long-term well- being. Lumbar puncture is effective in diagnosing ABM and is therefore a standard procedure in differential diagnosis when a patient presents with a first complex febrile seizure.10 However, lumbar puncture is invasive and can be traumatic, and there may be populations of patients that have a lower probability of serious neurological disease.10 Sales et al5 show a wide discrepancy of treatment plans between treating pediatric emergency physicians for patients presenting with complex febrile seizures. To better streamline patient care in a rushed pediatric ED, it’s important to establish which patients may require lumbar puncture more urgently than others.
In our sample of 193 patients, only 1 was subsequently diagnosed with ABM. The incidence rate of ABM in our population is comparable to that of similar studies, and supports the assertion that ABM is uncommon in this population.6,17 Therefore, it may be possible to identify a subset of patients presenting with a first complex febrile seizure who tend to be at lower risk of ABM or other serious neurological disease in order to administer appropriate treatment more efficiently.
Our analysis of the commonalities among patients who did receive lumbar puncture in our ED showed that these patients often presented with focality, status epilepticus, or a need for intubation. Seizure focality as a result of fluid accumulation in the subdural space may be an important indicator of ABM.11Convulsive status epilepticus has been found to be associated with increased rates of ABM.12 Intubation rates for pediatric patients presenting with seizures vary, but have been reported up to nearly 50%, and clearly indicate a need for immediate medical attention.13–16 These are not necessarily indications for lumbar puncture, but provide concrete evidence for the necessity of immediately ruling out bacterial meningitis or other neurological disease in these patients. While this may verge on common sense, the value of trained physicians’ clinical bias should not be overlooked. With this in mind, our sub-population analysis of patients presenting with 2 brief seizures within 24 hours excluded any patients with focality or status epilepticus by definition, and none of the eligible patients required intubation during their treatment in our ED. The tendency of pediatric emergency physicians to treat patients with these specific clinical indicators of neurological disease reflects an existing perception of what serious neurological disease looks like upon presentation to a pediatric ED. In contrast, our subpopulation may essentially represent the other end of the spectrum.
Our sub-population analysis included 43 patients, 17 of whom received lumbar puncture. No neurological disease was diagnosed in this group of patients, which is consistent with analyses in similar populations.6 None of the patients in this population were found to have neurological sequelae in follow up. While each febrile seizure should be evaluated on a case-by-case basis, our data suggest that patients who fall into this sub-population may be at a lesser risk of ABM or other serious neurological disease. Patients with 2 short febrile seizures within 24 hours without other signs of neurological disease may not require lumbar puncture in the pediatric ED.
LIMITATIONS
Retrospective analyses often include several limitations. One important factor to consider in our analysis is that ABM rates are relatively rare, so studies of its incidence in a given population require a very large sample size. It is difficult to make any strong conclusions on the rates of ABM within this population of 193 patients; we can simply note that our findings appear to be consistent with similar studies and hope to supplement the existing literature on this topic.
Another consideration is that Haemophilus influenzae type B vaccines are usually administered in young children before the age of 6 months to prevent bacterial meningitis.4 Status of this vaccine was not reported in patient charts, so we were unable to control for patients who had previously been vaccinated.
A final limitation was the use of ICD-9/10 codes for determination of our original subject list. Although we manually screened these patients’ records to ensure that each met eligibility requirements, other children experiencing FCFS may have been miscoded. This may particularly apply to patients experiencing multiple febrile seizures within the duration of a febrile illness where no other complex feature was observed, as each seizure event may have appeared to be a simple febrile seizure. We attempted to control for this by ensuring that our search for patient charts was as comprehensive as possible. We also assume that all complex features of the seizures were observed. This may not have been the case for children presenting with multiple seizures at home before presentation in the ED, or for patients who presented with focality that was not immediately recognized by the parent. These limitations are inevitable for this type of study. Future studies on this topic may focus on prospectively enrolling patients to gain a comprehensive understanding of febrile seizure presentation.
CONCLUSION
ABM is rare in patients presenting with a first complex febrile seizure. As Kimia et al6 have suggested, patients presenting only with 2 short febrile seizures within 24 hours may be less likely to have ABM, and may not require lumbar puncture without other clinical symptoms of neurological disease. Furthermore, in patients with first complex febrile seizure, lumbar puncture is significantly more likely to be performed on patients that presented with seizure focality, status epilepticus, or a need for intubation.
Footnotes
Supervising Section Editor: Mark I. Langdorf, MD, MHPE
Submission history: Submitted March 1, 2012; Revisions received July 17, 2012, Accepted August 13, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.8.12872
Address for Correspondence: Erin M. Fletcher, 3020 Children’s Way
MC 5075, San Diego, CA 92123. Email: emfletcher@gmail.com.
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Teng D, Dayan P, Tyler S. Risk of intracranial pathologic conditions requiring emergency intervention after a first complex febrile seizure episode among children. Pediatrics. 2006 Feb; 117(2):304–308. et al.[PubMed]
2. National Institute of Neurological Disorders and Stroke. Febrile Seizures Fact Sheet. National Institutes of Health. Available at:http://www.ninds.nih.gov/disorders/febrile_seizures/detail_febrile_seizures.htm. Accessed October 12, 2011.
3. Berg AT, Shinnar S. Complex Febrile Seizures. Epilepsia. 1996 Feb; 37(2):126–133. [PubMed]
4. Subcommittee on Febrile Seizures: American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011 Feb; 127(2):389–94. [PubMed]
5. Sales JW, Bulloch B, Hostetler MA. Practice variability in the management of complex febrile seizures by pediatric emergency physicians and fellows. CJEM. 2011 May; 13(3):145–149. [PubMed]
6. Kimia A, Ben-Joseph EP, Rudloe T. Yield of lumbar puncture among children who present with their first complex febrile seizure. Pediatrics. 2010 Jul; 126(1):62–69. et al. [PubMed]
7. Batra P, Gupta S, Gomber S. Predictors of meningitis in children presenting with first febrile seizures.Pediatr Neurol. 2011 Jan; 44(1):35–39. et al. [PubMed]
8. Seltz LB, Cohen E, Weinstein M. Risk of bacterial or herpes simplex virus meningitis/encephalitis in children with complex febrile seizures. Pediatr Emerg Care. 2009 Aug; 25(8):494–497. [PubMed]
9. Treatment of convulsive status epilepticus. Recommendations of the Epilepsy Foundation of America’s Working Group on Status Epilepticus. JAMA. 1993 Aug; 270(7):854–859. [PubMed]
10. World Health Organization. World Health Organization Media Centre; Meningococcal meningitis. Available at: http://www.who.int/mediacentre/factsheets/fs141/en/. Accessed October 12, 2011.
11. Green SM, Rothrock SG, Clem KJ. Can Seizures be the sole manifestation of meningitis in febrile children? Pediatrics. 1993 Oct; 92(4):527–534. et al. [PubMed]
12. Chin RF, Neville BG, Scott RC. Meningitis is a common cause of convulsive status epilepticus with fever. Arch Dis Child. 2005 Jan; 90(1):66–69. [PMC free article] [PubMed]
13. Stewart WA, Harrison R, Dooley JM. Respiratory depression in the acute management of seizures.Arch Dis Child. 2002 Sep; 87(3):225–226. [PMC free article] [PubMed]
14. Chiulli DA, Terndrup TE, Kanter RK. The influence of diazepam or lorazepam on the frequency of endotracheal intubation in childhood status epilepticus. J Emerg Med. 1991 Jan–Apr;9(1–2):13–17.[PubMed]
15. Babbitt CJ, Halpern R. Pediatric Status Epilepticus and Intubation. The Internet Journal of Pediatrics and Neonatology. 2005;4(2)
16. Singhi P, Singhi S. Neurocysticercosis in children. J Child Neurol. 2004 Jul; 19(7):482–492.[PubMed]
17. Bauchner H, Bakes K. Risk for Acute Bacterial Meningitis in Children with Complex Febrile Seizure.Journal Watch Pediatrics and Adolescent Medicine. 2010 Jul. Available at:http://pediatrics.jwatch.org/cgi/content/full/2010/728/1. Accessed October 12, 2011.
18. Consensus statement. Febrile seizures: long-term management of children with fever-associated seizures. Pediatrics. Dec 1980; 66(6):1009–1012. [PubMed]


