| Author | Affiliation |
|---|---|
| Jason Bothwell, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| Carl Skinner, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| David Della-Giustina, MD | Yale School of Medicine, Department of Emergency Medicine, New Haven, Connecticut |
| Christopher Kang, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| Laura Cookman, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
| Brooks Laselle, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, Washington |
Introduction
Methods
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
Acute toxic ingestion is a common cause of morbidity and mortality. Emergency physicians (EP) caring for overdose (OD) patients are often required to make critical decisions with incomplete information. Point of care ultrasound (POCUS) may have a role in assisting EPs manage OD patients. We evaluated the impact of different liquid adjuncts used for gastric decontamination on examiners’ ability to identify the presence of tablets using POCUS, and assessed examiners’ ability to quantify the numbers of tablets in a simulated massive OD.
Methods
This prospective, blinded, pilot study was performed at an academic emergency department. Study participants were volunteer resident and staff EPs trained in POCUS. Five nontransparent, sealed bags were prepared with the following contents: 1 liter (L) of water, 1 L of water with 50 regular aspirin (ASA) tablets, 1 L of water with 50 enteric-coated aspirin tablets (ECA), 1 L of polyethylene glycol (PEG) with 50 ECA, and 1 L of activated charcoal (AC) with 50 ECA. After performing POCUS on each of the bags using a 10-5 MHz linear array transducer, participants completed a standardized questionnaire composed of the following questions: (1) Were pills present? YES/NO; (2) If tablets were identified, estimate the number (1–10, 11–25, >25). We used a single test on proportions using the binomial distribution to determine if the number of EPs who identified tablets differed from 50% chance. For those tablets identified in the different solutions, another test on proportions was used to determine whether the type of solution made a difference. Since 3 options were available, we used a probability of 33.3%.
Results
Thirty-seven EPs completed the study. All (37/37) EP’s correctly identified the absence of tablets in the bag containing only water, and the presence of ECA in the bags containing water and PEG. For Part 2 of the study, most participants – 25/37 (67.5%) using water, 23/37 (62.1%) using PEG, and all 37 (100%) using AC – underestimated the number of ECA pills in solution by at least 50%.
Conclusion
There may be a potential role for POCUS in the evaluation of patients suspected of acute, massive ingested OD. EPs accurately identified the presence of ECA in water and PEG, but underestimated the number of tablets in all tested solutions.
INTRODUCTION
Background
Acute toxic ingestion is a common cause of morbidity and mortality. In 2011 the Annual Report of the American Association of Poison Control Centers’ National Poison Data System recorded more than 2.3 million accidental or intentional toxic exposures and 1,158 deaths.1 Emergency physicians (EP) caring for overdose (OD) patients are often required to make critical decisions with incomplete information regarding the substance(s), quantity, or time elapsed since ingestion. In cases of suspected overdose, patients may be obtunded and unable to provide essential information or may withhold details of the ingestion.
Importance
Due to this uncertainty, EPs often rely on diagnostic testing to supplement their clinical suspicion. This may involve laboratory analysis for serum drug concentrations or imaging for the identification of pills in the stomach. Both plain radiography and computed tomography have been described, but neither is reliable and both involve radiation exposure.2,3 Furthermore, neither can be performed in real time at the bedside by the treating physician. A few case reports and one small prospective trial describe ultrasound for identification of pills in the stomach.4–7 However, there are no published data on the ability of practitioners to visualize ingested tablets during decontamination therapy with activated charcoal or polyethylene glycol, both of which are commonly administered to overdose patients. This is of potential consequence in pre-hospital care situations, where some protocols initiate decontamination therapy prior to hospital arrival.
The massive OD presents a unique problem for EPs. Not only do patients have the potential to rapidly decompensate, but many medications often believed to be relatively benign can have devastating consequences. Some experts recommend more aggressive hemodialysis for suspected massive overdoses, as gastric decontamination parameters may be altered.8 In these cases, any test that successfully identifies the massive OD could potentially alter management and outcome. As this time, there are no prospective studies evaluating EPs’ ability to quantify pills in a suspected massive overdose.
Study Objectives
The primary objective of this feasibility study was to evaluate the impact of the presence of different liquid adjuncts used for gastric decontamination on examiners’ ability to identify the qualitative presence of tablets using ultrasound. A secondary objective was to assess examiners’ ability to quantify the numbers of tablets in a simulated massive overdose.
METHODS
Study Design and Selection of Participants
This prospective, blinded pilot study was approved by the institutional review board. Study participants were volunteer emergency medicine resident and staff physicians from an academic emergency department. Eligible participants previously completed a minimum 2-day ultrasound training course consisting of 16 hours of basic and advanced point of care ultrasound applications. An emergency medicine intern performed recruitment to avoid any appearance of coercion, and provided an information and “opt out” sheet. As there was no risk to participants, informed consent was waived. Prior to participation, all participants watched a training video demonstrating tablets under ideal sonographic conditions. The video consisted of a 6-second clip showing 50 enteric-coated aspirin in 1 liter of water. (Participants were not informed of which tablet or substance was represented in the video clip.
To compare the visibility of a simulated massive OD of tablets in 3 different media, 5 sealed bags were prepared with the following contents: 1 liter of water, 1 liter of water with 50 regular aspirin (ASA) tablets, 1 liter of water with 50 enteric-coated aspirin tablets (ECA), 1 liter of polyethylene glycol (PEG) with 50 ECA, and 1 liter of activated charcoal (AC) with 50 ECA.
The contents were placed in black, opaque plastic sealable bags, thus blinding the participants to the contents. Prior to sealing, bags were manually squeezed to eliminate air. Bags were placed in random order on a table in a linear arrangement. One at a time, participants performed ultrasounds on the bags using a Zonare One™ (Zonare Medical Systems, Mountain View CA) ultrasound machine with a 10-5 MHz linear array transducer. No restrictions were imposed on the order of bags scanned, the number of scans per bag or scanning time. The study took place in 3 separate sessions, 1 week apart, with random numbering for each bag prior to each session. No bag was used for longer than 1 hour, to minimize the effects of pills dissolving during the study.
Data Collection and Processing
After scanning, participants filled out a brief, standardized questionnaire responding to the following questions: (1) Were pills present? YES/NO; (2) If pills were identified, estimate the number (1–10, 11–25, >25). The primary investigator manually transferred data into an electronic spreadsheet Microsoft Excel 2007™ (Microsoft Corporation, Redmond, WA) at the conclusion of each session, and one of the associate investigators then independently the data.
Outcome Measures
The primary outcome was the ability to detect the qualitative presence of pills in water and in the different gastric decontamination media (PEG and AC). The secondary outcome was the ability of the participants to accurately estimate the number of pills in solution.
Primary Data Analysis
All data were analyzed using Microsoft Excel 2007™ (Microsoft Corporation, Redmond, WA). We used a single test on proportions using the binomial distribution to determine if the number of physicians who saw tablets differed from 50% chance. For those tablets that were identified in the different solutions, we used another test on proportions to determine whether the type of solution made a difference. Since there were 3 options from which to choose, we used a probability of 33.3% in the calculation.
RESULTS
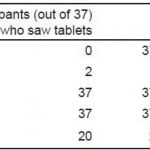
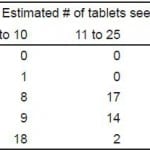
A total of 37 physicians completed the study, and no participant required more than 5 minutes. The number of physicians who visualized tablets in various solutions is shown in Table 1. For those physicians who saw (any) tablets in a solution, the number they estimated for that solution is shown in Table 2. For Part 1 of the study, all (37/37) study participants correctly identified:
Table 1. Participants’ visualization of tablets in different solutions.
ASA, aspirin; ECA, enteric-coated aspirin
Table 2. Estimated number of tablets visualized in various solutions.
ASA, aspirin; ECA, enteric-coated aspirin
- the presence of ECA in the bag containing water
- the presence of ECA in the bag containing PEG
All 37/37 (100%) participants were also accurate in determining the absence of ECA tablets in a water-only model where pills were non-existent, and 35/37 (95%) did not visualize ASA in water. For Part 2 of the study, most participants — 25/37 (67.5%) using water, 23/37 (62.1%) using PEG, and all 37 (100%) using AC — underestimated the number of ECA pills in solution by at least 50%.
DISCUSSION
This simple bag model suggests that point of care ultrasound is potentially useful for detecting the presence of tablets in a massive OD, but less useful for quantifying them. Participants were accurate in determining the absence of tablets in a water-only model and in accurately determining the presence of ECA in both water and in PEG. This suggests that ECA is readily visualized in these solutions with ultrasound by EPs.
AC appears to substantially interfere with the sonographic visualization of ECA, given that only 20/37 (54%) correctly identified the presence of ECA tablets in the bag containing AC. This was not significantly different from random chance (p=0.116). When tablets were visualized in AC, the estimated number was very low compared to the total number of pills present. If ultrasound is used clinically, it should be noted that any AC impairs accurate visualization. Participants were also generally unable to identify regular ASA in water (2/37, 5%), likely because the tablets had dissolved. In our own experience with this experiment using clear plastic bags, regular ASA dissolves within 60 seconds when placed in room-temperature water. This suggests that the use of ultrasonography to detect regular aspirin tablets may not be feasible. ECA, however, remains intact and visible with ultrasound in solution for well over an hour, with only minor flaking of the coating.
Regardless of the solution tested, the majority of participants substantially underestimated the number of tablets when they were present. Even though each tablet-containing bag held 50 tablets, only 12/37 (32%) of participants estimated more than 25 tablets in water, and 14/37 (38%) estimated more than 25 tablets in PEG. For the tablets in AC, none of the participants estimated greater than 25 tablets. Applying this to an actual patient-care situation, any tablets visualized on ultrasound in a human stomach would potentially be an underrepresented of the total number present, and any search for tablets in a suspected OD should be performed prior to the administration of AC. As always, the clinical presentation of the patient would factor into the interpretation of any ultrasound images. However, it appears that underestimation would be the most common error in quantifying the size of the ingestion.
This is the first controlled trial, to our knowledge, that examines the effects of gastric decontamination solutions on the accuracy of diagnostic ultrasound. It is also the first study to evaluate participants’ ability to quantify tablets in solution. The use of ultrasound for detection of pills in the stomach was first described in the late 1980s,5 but there have been few studies since then expanding on this early foundation of knowledge. Our study suggests the feasibility of ultrasound as a tool for identifying ECA tablets in certain solutions. Clarifying the role of point of care sonography in massive OD requires further investigation. This study, performed in artificial but sonographically ideal conditions, needs validation in human subjects. Other warranted investigations include the sonographic properties of capsules, sustained- release preparations, other enteric-coated medications, and whether sonographic properties vary between medications or due to the specific coatings applied to those medications.
The use of point of care ultrasound has increased substantially in recent years. As outlined in the most recent ultrasound guidelines from the American College of Emergency Physicians a number of applications are “evolving.”9 As EPs expand their familiarity with ultrasound technology, it is likely that the list of applications will expand as well, possibly to include gastric ultrasound for pill identification in cases of massive ingestion.
LIMITATIONS
Only 2 types of tablets were evaluated in this study: regular ASA and ECA. Accordingly, these results may not be applicable to other medications or formulations. We used 50 tablets to simulate a “massive overdose.” This number can be debated and clearly varies based on the medication ingested. However we feel this number is a reasonable estimate of a fatal ingestion for over-the-counter medications that are readily available and commonly consumed during intentional ingestions. Finally, this in vitro study using bag models and non-physiologic solutions for a simulated massive OD cannot be extrapolated to human subjects. It is expected that visualizing pills in a plastic bag would be easier than doing so under normal clinical conditions.
CONCLUSION
There may be a potential role for point of care ultrasound in the evaluation of the acutely poisoned patient. We found that regular ASA in solution is difficult to identify with ultrasound, but that ECA is easily identified in water and in PEG. AC, however, appears to substantially impair visualization of ECA. We also discovered that EPs are likely to significantly underestimate the number of tablets present. Further studies will be required to evaluate and validate the potential role for this application in patients with acute ingestions.
Footnotes
Address for Correspondence: Jason Bothwell, MD, Department of Emergency Medicine, Madigan Army Medical Center, 3844 Long Lake Loop SE, Lacey, WA 98503. Email: jasonbothwell@yahoo.com. 3 / 2014; 15:176 – 179
Submission history: Revision received May 17, 2013; Submitted August 24, 2013; Accepted October 28, 2013
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The views expressed are those of the author(s) and do not reflect the official policy of the Department of the Army, the Department of Defense or the United States Government.
REFERENCES
1. Bronstein AC, Spyker DA, Cantilena LR, et al. 2011 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th Annual Report. Clin Toxicol. 2011;49:910-941.
2. Savitt DL, Hawkins HH, Roberts JR. The Radiopacity of Ingested Medications. Ann Emerg Med. 1987;16:331-339.
3. O’Brien RP, McGeehan PA, Helmeczi AW, et al. Detectability of Drug Tablets and Capsules by Plain Radiography. Am J Emerg Med. 1986;4:302-312.
4. Amitai Y, Silver B, Leikin J, et al. Visualization of Ingested Medications in the Stomach by Ultrasound. Am J Emerg Med. 1992;10:18-23.
5. Maublant JC, Hassine H, Sournac M, et al. Ultrasonic Visualization of Tablets in the Gastrointestinal Tract. J Nuc Med. 1988;29:129.
6. Schlager D. Ultrasound detection of foreign bodies and procedure guidance. Emerg Med Clin North Am. 1997;15:895-912.
7. Nordt SP, Campbell C, Medak A, et al. Ultrasound Visualization of Ingested Tablets: A Pilot Study. Pharmacotherapy. 2011; 31:273-276.
8. Erickson T. The New Overdoses. Course lecture presented at: American College of Emergency Physicians, Scientific Assembly, October 2008; Chicago, IL.
9. American College of Emergency Physicians Policy Statement: Emergency Ultrasound Guidelines. October, 2008.