| Author | Affiliation |
|---|---|
| Yu-Hsiang Hsieh, PhD | Johns Hopkins University School of Medicine, Department of Emergency Medicine, Baltimore, Maryland |
| Gabor D. Kelen, MD | Johns Hopkins University School of Medicine, Department of Emergency Medicine, Baltimore, Maryland |
| Andrea F. Dugas, MD | Johns Hopkins University School of Medicine, Department of Emergency Medicine, Baltimore, Maryland |
| Kuan-Fu Chen, MD, PhD | Johns Hopkins University School of Medicine, Department of Emergency Medicine, Baltimore, Maryland Chang Gung Memorial Hospital, Department of Emergency Medicine, Taoyuan, Taiwan Chang Gung University, Taoyuan, Taiwan |
| Richard E. Rothman, MD | Johns Hopkins University School of Medicine, Department of Emergency Medicine, Baltimore, Maryland |
ABSTRACT
Introduction:
Little is known regarding compliance with management guidelines for epidemic influenza in adult emergency department (ED) settings during the 2009 novel influenza A (H1N1) epidemic, especially in relation to the Centers for Disease Control and Prevention (CDC) guidance.
Methods:
We investigated all patients with a clinical diagnosis of influenza at an inner-city tertiary academic adult ED with an annual census of approximately 60,000 visits from May 2008 to December 2009. We aimed to determine patterns of presentation and management for adult patients with an ED diagnosis of influenza during the H1N1 pandemic, using seasonal influenza (pre-H1N1) as reference and to determine the ED provider’s adherence to American College of Emergency Physicians and CDC guidance during the 2009 H1N1 influenza pandemic. Adherence to key elements of CDC 2009 H1N1 guidance was defined as (1) the proportion of admitted patients who were recommended to receive testing or treatment who actually received testing for influenza or treatment with antivirals; and (2) the proportion of high-risk patients who were supposed to be treated who actually were treated with antivirals.
Results:
Among 339 patients with clinically diagnosed influenza, 88% occurred during the H1N1 pandemic. Patients were similarly managed during both phases. Median length of visit (pre-H1N1: 385 min, H1N1: 355 min, P > 0.05) and admission rates (pre-H1N1: 8%, H1N1: 11%, P > 0.05) were similar between the 2 groups. 28% of patients in the pre-H1N1 group and 16% of patients in the H1N1 group were prescribed antibiotics during their ED visits (P > 0.05). There were 34 admitted patients during the pandemic;, 30 (88%) of them received influenza testing in the ED, and 22 (65%) were prescribed antivirals in the ED. Noticeably, 19 (56%) of the 34 admitted patients, including 6 with a positive influenza test, received antibiotic treatment during their ED stay.
Conclusion:
During the recent H1N1 pandemic, most admitted patients received ED diagnostic testing corresponding to the current recommended guidance. Antibiotic treatment for ED patients admitted with suspected influenza is not uncommon. However, less than 70% of admitted patients and less than 50% of high-risk patients were treated with antivirals during their ED visit, indicating a specific call for closer adherence to guidelines in future influenza pandemics.
INTRODUCTION
The unexpected emergence of swine-origin novel influenza A (H1N1) virus in the early spring of 2009 spread rapidly across North America and to the rest of the world, followed by a second wave in the fall. It is estimated that there were 39–80 million cases, 173,000–362,000 hospitalizations and 7,880–16,460 deaths in the United States (U.S.) alone as of mid-December 2009 and approximately 300,000 deaths worldwide.1–3 Due to the acute and occasionally severe nature of influenza viral infections, emergency departments (EDs) often serve as the frontline for infected patients, especially among children and the elderly.4 Based on a nationally representative survey study, an estimated 0.3% or 312,000 of all-aged ED visits received a diagnosis of influenza annually from 2002 to 2006.5Approximately 40% of visits had antiviral prescriptions provided during their ED visits. During the influenza seasons, the ED may become filled with influenza-like illness (ILI) patients, making it a high-risk medical venue for nosocomial transmission of influenza.6,7
The Centers for Disease Control and Prevention (CDC) held the first press briefing to inform the media and guide the public and healthcare response to the novel H1N1 influenza virus on April 23, 2009 after the novel virus was first detected and confirmed by the CDC 8 days prior. This was followed by multiple additional cases identified in several states, as well as in Mexico over the next few days.8 In response to the emergence of novel H1N1 influenza, both the American College of Emergency Physicians (ACEP) 9,10 and the CDC 11–16 prepared and distributed guidelines and recommendations for clinicians addressing diagnostic testing and antiviral treatment for clinical providers in ED settings for patients with suspected or confirmed influenza in early May 2009 (Table 1; Figure).8,10 Most key elements from the 2 organizations were similar.
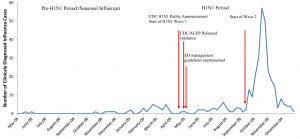
Weekly number of adult emergency department patients given a diagnosis of influenza from May 2008 to December 2009 at Johns Hopkins Hospital.
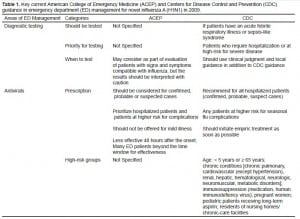
Key current American College of Emergency Medicine (ACEP) and Centers for Disease Control and Prevention (CDC) guidance in emergency department (ED) management for novel influenza A (H1N1) in 2009.
Little is known regarding compliance with management guidelines for epidemic influenza in adult ED settings during the novel H1N1 epidemic, especially in relation to current ACEP and CDC guidance. Understanding patterns of clinical presentation provides baseline data for future pandemic preparedness efforts, while adherence to current clinical management recommendations for patients with a clinical diagnosis of influenza in the ED helps address potential areas for improved adherence. Thus, this study aimed (1) to determine patterns of presentation and management for adult patients with an ED diagnosis of influenza during the H1N1 pandemic using seasonal influenza (pre-H1N1) as reference (where 2008–09 influenza season has a relative mild season)17 and (2) to determine the ED providers’ adherence to ACEP and CDC guidance during the 2009 H1N1 influenza pandemic.
METHODS
We conducted an analysis of all patient visits to an inner-city tertiary academic adult ED that had an annual census of approximately 60,000 visits from May 1, 2008 to December 31, 2009. Hospital Epidemiology & Infection Control (institutional level) and the Adult ED Influenza Administrative Cabinet (departmental level) operationalized novel H1N1 strategic plans designed to synchronize, as much as possible, with the 2009 CDC and ACEP guidance, as it evolved over the course of the pandemic.9–16 The only key difference between the ED guidelines and CDC/ACEP guidelines was in diagnostic testing. Our institution recommended ED patients being admitted with suspected influenza should be tested for novel influenza virus, while CDC recommended clinicians should test patients with priority given to both those being admitted and those at a higher risk for complications (Table 1). Initial hospital and departmental training for the response to 2009 H1N1 occurred within 1–2 weeks of CDC and ACEP guidelines release in early May 2009 (Figure). More frequent and intensive trainings and interventions were carried out during the dramatic surge of the second wave of H1N1 pandemic in late September 2009. The plans were disseminated to all ED providers via broadcast emails, internal websites and town hall meetings, and were revised according to changing CDC guidance, necessitated by the changing understanding of the pandemic itself. This study protocol was approved by institutional review board of The John Hopkins University School of Medicine.
Data were captured and queried from an ED electronic patient record system, including demographics (age, gender, and race), ED presentation (chief complaint, acuity level of triage severity18, onset of illness), ED management (chest radiograph, influenza testing, nasopharyngeal specimen collection, nebulized medication, intubation, antibiotic prescription, type of antibiotic prescribed, antiviral prescription, duration of ED visit, and disposition) and final ED diagnosis. Additional laboratory influenza virus typing data and clinical data regarding co-morbid conditions were chart-reviewed and abstracted from the electronic patient record system, specifically including chronic pulmonary, cardiovascular (except hypertension), renal, hepatic, hematological, neurologic, neuromuscular, metabolic disorders, immunosuppression status (medication, human immunodefiency virus [HIV]), pregnancy, and residence in nursing homes or chronic-care facilities. Following appropriate training for this project, chart review and data abstraction was performed by one co-author (K-F C) who is an emergency medicine attending physician.
“Patients with a clinical diagnosis of influenza” in this study was operationally defined as a visit with any ED discharge diagnosis of influenza, i.e. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 487, 487.0, 487.1 or 487.8. To strictly evaluate provider’s adherence to CDC guidance during the 2009 H1N1 pandemic, a patient with an ambiguous indication or diagnosis of ILI was not considered as a cases of influenza. A subsequent visit with an ED diagnosis of influenza was excluded if the later visit occurred < 1 week from the initial visit. Patients were categorized into pre-H1N1 (May 1, 2008 through April 22, 2009; 11.7 months) and H1N1 groups (April 23, 2009 through December 31, 2009; 8.3 months) according to the CDC announcement of the H1N1 outbreak. H1N1 group was further categorized to H1N1 Wave 1 (April 23, 2009 through mid-September 2009) and Wave 2 (late September 2009 through December 31, 2009) according to the epidemic of H1N1 in U.S. (Figure). CDC recommendations on diagnostic testing and antiviral treatment for admitted and high-risk group patients during pre-H1N1 seasonal influenza were highly similar to those during novel H1N1 pandemic. Direct immunofluorescence assays (DFA, D3 ultra DFA respiratory virus ID kit, Diagnostic Hybrids, Athens, Ohio, U.S.), which had a sensitivity of 93% for the detection of 2009 novel H1N1 virus,19 and/or culture (shell vial & conventional) were the main diagnostic tests used for influenza by the clinical virology laboratory during the study period as the virology director removed the rapid influenza test (Binax) from use on April 28, 2009 due to poor sensitivity, as low as 11%.20 Any specimen that was H1N1 positive during the H1N1 period was sent to the state laboratory for confirmation of novel H1N1 virus by reverse transcription polymerase chain reaction.
During the H1N1 period, high risk groups for diagnostic testing and antiviral prescription were defined according to CDC guidance for antiviral prescription (Table 1). Basic adherence to key elements of ACEP or CDC 2009 H1N1 guidance was operationally defined as (1) the proportion of admitted patients who were supposed to be tested or treated who actually were tested for influenza or treated with antivirals; and (2) the proportion of high-risk patients who were supposed to be treated who actually were treated with antivirals.
We performed descriptive analysis, followed by chi-square test and Fisher’s exact test for categorical data or Wilcoxon rank sum test for continuous data to compare patterns of presentation and care between pre-H1N1 and H1N1 groups and ED management between high risk and non-high risk groups. All statistical analyses were performed by using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
During the 20-month study period, there were 103,417 patient visits encountered in this inner-city academic adult ED, and of these, 45,881 (44%) visits were during H1N1 period. Overall, 339 patient visits from 331 patients were clinically diagnosed with influenza. Of these, 300 (88%) occurred during the H1N1 outbreak (Figure), an approximately 10-fold increase in the proportion of clinically diagnosed influenza cases (65.0 per 10,000 visits versus 6.8 per 10,000 visits). All 8 influenza repeated visits occurred during the outbreak. Three repeated visits had < 7-day interval after the initial visit.
Comparing the pre-H1N1 and H1N1 group there were no differences in patients’ demographics or proportions of high-risk groups as designated by ACEP and CDC (Table 2). More patients in the H1N1 group reported “flu” as their chief complaint, versus those in pre-H1N1 group (P < 0.05), but there were no statistical differences between the 2 groups in other common chief complaints for influenza patients. Patients in the H1N1 group had a higher triage acuity, i.e. level 1–3, which reflected higher severity of disease (P < 0.05). In both groups, approximately 10% of those who received chest radiograph had confirmed pneumonia (12.1% versus 10.3%, P = 0.787). Similar proportions of patients in both groups had nasopharyngeal specimens collected for flu tests. Overall, among 110 patients who received a test, 55 (50%) were positive for influenza with 8 detected by the rapid influenza test. The majority (70%) of positives in the pre-H1N1 group (n = 10) were influenza A followed by 30% with influenza B, while all of the positives in the H1N1 group (n = 45) were influenza A, with one third (33.3%) of these confirmed as novel H1N1.
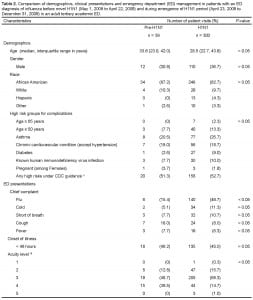
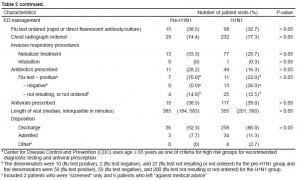
Regarding antibiotic prescription in the ED for these clinically diagnosed influenza patients, we found that 28% of patients in the pre-H1N1 group and 16% of patients in the H1N1 group were prescribed antibiotics during their ED visits (P > 0.05). Further analysis by influenza test results identified that 70% (7/10) patients who tested positive received antibiotics in the pre-H1N1 group, which was statistically significantly higher than the 22% (11/50) who tested positive in the H1N1 group (P < 0.05) (Table 2). During the H1N1 pandemic, a significantly higher proportion of patients who tested negative for influenza received an antibiotic prescription than those who did not have the test or whose results were not available (26.0% versus 12.5% P < 0.05) (Table 2). Azithromycin (n = 35) was the leading antibiotic prescribed to patients with a clinical diagnosis of influenza, followed by moxifloxacin (n = 15). Noticeably, 19 (56%) of 34 admitted patients during the 2009 novel H1N1 epidemic, including 6 influenza tested positive patients, received antibiotic treatment during their ED stay for community-acquired pneumonia. The list of antibiotics prescribed in ED for these 19 patients is summarized in Table 3. For those 6 patients who were tested positive for influenza and received antibiotic treatment, none had a pulmonary infiltrate on their chest radiograph; 3 received antivirals and 3 did not (all 3 having > 48 hours onset of illness); 1 with asthma had a final discharge diagnosis of pneumonia, 2 were HIV-infected, 2 had asthma and 1 did not have any high risk underlying illness on the chart.
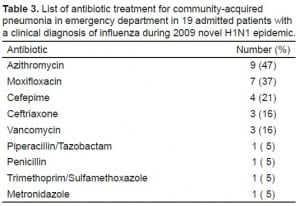
List of antibiotic treatment for community-acquired pneumonia in emergency department in 19 admitted patients with a clinical diagnosis of influenza during 2009 novel H1N1 epidemic.
Treatment rates with antivirals were similar at 39%, with oseltamivir given in 93% of those receiving antivirals during pre-H1N1 and 100% during H1N1 period. For 153 patients with onset of illness < 48 hours, 42% of them received antivirals, which had no statistical difference as compared to those 186 patients without (37%, P > 0.05). Median length of visit (pre-H1N1: 385 min, H1N1: 355 min) and admission rates (pre-H1N1: 8%, H1N1: 11%) were similar between the 2 groups. There was one patient deceased during hospitalization in each phase. The overall mortality rate was not statistically different (pre-H1N1: 2.6% versus H1N1: 0.3%, P = 0.231).
Regarding ED provider’s adherence to CDC/ACEP guidance in influenza diagnostic testing and antiviral treatment during H1N1 pandemic, we found that 88% of admitted patients received a testing order, 49% of high-risk group patients were prescribed with antivirals, and 65% of admitted patients were prescribed with antivirals (Table 4). No significant uniform trends in adherence were observed during the study period. If only focusing on admitted patients, 67% were ordered for influenza testing during pre-H1N1, 100% during H1N1 wave 1, and 87% during wave 2. The proportion of high risk group patients who were prescribed antiviral treatment during their ED stay gradually increased from 30% during pre-H1N1 to 39% during H1N1 wave 1, and further increased to 51% during wave 2 (Cochran-Armitage trend test, P = 0.059). Among admitted patients, 67% were prescribed with antivirals during pre-H1N1, none of 4 patients during H1N1 wave 1, but significantly increased back to 73% during wave 2 (P < 0.05).
DISCUSSION
To our knowledge, there are few studies to date examining ED care and management for adult patients with a discharge diagnosis of influenza before and during the H1N1 epidemic, as well as rates of ED provider adherence with the 2009 ACEP and CDC guidance (for patients with suspected H1N1). Although the demographic profiles of patients pre and during the H1N1 were similar, our results reveal that significantly different patterns of clinical presentation (including chief complaint and level of acuity) emerged during the H1N1 epidemic. Published studies, which included adult ED encounters, demonstrated some differences in symptom patterns when comparing patients of pandemic novel H1N1 and seasonal influenza.21,22 Tang et al21 reported a lower incidence of fever and dyspnea in early H1N1 pandemic in Singapore, while Shiley et al22 documented cough and myalgias were more common in patients with a diagnosis of pandemic H1N1 at 2 medical centers in Philadelphia. Our findings that rates of hospital admission did not differ between the pre H1N1 and post H1N1 groups is consistent with U.S. CDC surveillance data, which do not show an increase in pneumonia and influenza mortality after the emergence of H1N1.21
Although the recommendations of both ACEP and CDC with regard to diagnostic test ordering practices and antiviral prescriptions decisions are only suggestions to begin with, further exploration of ED management found that practices in this ED were in “very good” or “excellent” accordance in diagnostic testing but in “suboptimal” accordance in antiviral prescription with the 2009 ACEP and CDC recommendation. This finding suggests that ED practice yielded from “suboptimal” to “excellent” adherence to recommended care for high-risk patients in ordering diagnostic tests and antiviral prescriptions, even though the practice ultimately is at the discretion of the provider, but influenced by a combination of individual patient features and institutional and national guidance. The adherence in antiviral treatment was much better in more severe subgroup patients, i.e. admitted patients, during wave 2 of the H1N1 pandemic. This is partly supported by the finding from our subgroup analysis that only triage acuity and chief complaint were associated with a provider’s order for influenza diagnostic testing or antiviral treatment in high-risk group patients (data not shown). Coupled with our previous finding, which showed a 97% adherence with CDC interim guidelines for antiviral prescription from nationally representative survey data,5 this study suggests that ED clinicians are potentially appropriately responsive and could be adherent with national guidance for influenza, which will be critical for coping with future influenza pandemics. On the other hand, approximately less than 50% of high-risk patients were prescribed antivirals during their ED visits, indicating that there remains room for improvement with regard to adherence to recommended management strategies among ED clinicians. This is imperative since delays in testing and treatment can quickly lead to increases in preventable deaths due to influenza.23,24 Further studies are required to develop approaches to improve adherence for future, potentially more virulent pandemics, e.g. direct electronic reminders to providers as suggested by May et al.25
Antibiotic overuse for ED patients with acute respiratory infections is still substantial in the Unites States, even though there has been a downward trend in the recent years.26–29 According to previous work in clinically diagnosed influenza patients by Linder et al30, antibiotics were inappropriately prescribed to an overall 26% of ambulatory clinics and ED influenza visits (approximately 15% for ED). Our study found that 28% of patients in the pre-H1N1 group, 16% in the H1N1 group, and more than half of admitted patients with ED clinical diagnosis of influenza received antibiotics in the ED. The decreased antibiotic prescription rate seen during the H1N1 outbreak despite an increased patient acuity could represent increased provider confidence in the diagnosis of influenza or increased awareness of antibiotic overuse. Nevertheless, our findings imply that there is additional work needed for reducing potential antibiotic overuse in the ED.
LIMITATIONS
There are some limitations to this study. First, ED clinical diagnosis of influenza could be subjective and is without specific standard criteria. It is possible that some true influenza cases may have been missed during the pre-H1N1 mild influenza season and diagnosed as a viral syndrome, while some “clinically diagnosed” cases of pandemic H1N1 influenza during the more severe influenza season could have been respiratory illnesses from other causes. It is likely that heightened awareness of influenza during a pandemic increased the proportion of patients with ILI receiving a diagnosis of “influenza” rather than “viral illness,” or other nonspecific diagnoses. In addition, we do not have evidence that subgroups of clinical diagnosed influenza patients without laboratory-confirmed infection between 2 periods are similar. However, it is more appropriate to use clinically diagnosed influenza patients as our study subjects rather than more “loose” defined ILI patients in terms of understanding of ED provider’s adherence to ACEP and CDC guidance especially in regard to antiviral prescription variables. Second, our findings from this academic inner-city ED were specific to our ED and may not be generalizable to other EDs in U.S. For example, our clinically diagnosed influenza patients were much younger than those in the National Hospital Ambulatory Medical Care Survey in seasonal influenza seasons (median age: 33.6 years versus 41.5 years).5 However, our data reflect the general trend that young adults and adults are the main population attacked by the 2009 H1N1 pandemic.1 Finally, we did not account for the lag time between ACEP, CDC, institutional/departmental guidance and provider’s practice in our analysis. However, we expect that lag time should be minimal in future influenza pandemics, and that providers must be able to rapidly respond to up-to-date recommendations.
CONCLUSION
In summary, ED management with regard to diagnostic testing and antiviral prescription for admitted patients or those designated as high risk groups by ACEP and CDC ranged from “suboptimal” to “very good” or “excellent” in regard to current guidance during the pandemic. However, only 49% were treated with antivirals during their ED visits, indicating a specific call for closer adherence to guidelines in future influenza pandemics. Despite a decrease in antibiotic prescription rates during the H1N1 period, continued antimicrobial stewardship is required.
Footnotes
Supervising Section Editor: Robert Derlet, MD, MPH
Submission history: Submitted March 29, 2012; Revisions received September 14, 2012; Accepted November 21, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.11.12246
Address for Correspondence: Yu-Hsiang Hsieh, PhD, Johns Hopkins University School of Medicine, Department of Emergency Medicine, 1830 East Monument Street, Suite 6-100, Baltimore, MD 21287. Email: yhsieh1@jhmi.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed Drs. Hsieh, Rothman and Kelen are supported in part by a grant from the US Department of Homeland Security (Grant Number N00014-D6-1-0991).
REFERENCES
1. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood F, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med.2009;360:2605–2615. [PubMed]
2. CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April – December 12, 2009., 2010. Available athttp://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm. Accessed January 18, 2010.
3. Dawood F, Iuliano A, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis.2012;12:687–695. [PubMed]
4. Olson D, Heffernan R, Paladini M, et al. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLos Med. 2007;4:e247.[PMC free article] [PubMed]
5. Hsieh Y, Chen K, Gaydos C, et al. Antiviral Prescriptions to US Ambulatory Care Visits with a Diagnosis of Influenza Before and After High Level of Adamantane Resistance 2005–06 Season. PLoS One. 2010;5:e8945. [PMC free article] [PubMed]
6. Weingarten S, Friedlander M, Rascon D, et al. Influenza surveillance in an acute-care hospital.Arch Intern Med. 1988;148:113–116. [PubMed]
7. Rothman R, Hsieh Y, Yang S. Communicable respiratory threats in the ED: tuberculosis, influenza, SARS, and other aerosolized infections. Emerg Med Clin North Am. 2006;24:989–1017. [PubMed]
8. The 2009 H1N1 Pandemic: Summary Highlights, April 2009–2010. Rep. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/h1n1flu/cdcresponse.htm. Accessed August 27, 2012.
9. American College of Emergency Physicians; 2009. FAQ’s for Emergency Departments in Epidemic or Pandemic Influenza Outbreaks. Available at: http://www.acep.org/practres.aspx?LinkIdentifier=id&id=45423&fid=908&Mo=No. Accessed November 9, 2009.
10. National Strategic Plan for Emergency Department Management of Outbreaks of Novel H1N1 Influenza. American College of Emergency Physicians. 2009. Available athttp://www.acep.org/WorkArea/DownloadAsset.aspx?id=45781. Accessed November 9, 2009.
11. Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnel. Centers for Disease Control and Prevention (CDC), 2009. Available at http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed Febuary 4, 2010.
12. Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnelage. Rep. Centers for Disease Control and Prevention. Available at: www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed Febuary 4, 2010.
13. Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season. Centers for Disease Control and Prevention (CDC), 2009. Available at: www.cdc.gov/h1n1flu/recommendations.htm. Accessed Februay 4, 2010.
14. CDC Health Alert Network (HAN) Info Service Message: CDC Guidance on Antiviral Treatment of Patients with Confirmed, Probable, or Suspected Cases of Novel Influenza A (H1N1). 2009. Available at: http://www.cdc.gov/h1n1flu/HAN/052709.htm. Accessed February 4, 2010.
15. CDC Health Alert Network (HAN) Info Service Message: Recommendations for Early Empiric Antiviral Treatment in Persons with Suspected Influenza who are at Increased Risk of Developing Severe Disease Centers for Disease Control and Prevention (CDC), 2009. Available athttp://www.cdc.gov/H1N1flu/HAN/101909.htm. Accessed February 4, 2010.
16. Centers for Disease Control and Prevention (CDC); 2009. Interim Recommendations for Clinical Use of Influenza Diagnostic Tests During the 2009–10 Influenza Season. Available at:http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/08-09summary.htm. Accessed February 4, 2010.
17. Gilboy N, Tanabe P, Travers D, et al. Emergency Severity Index, Version 4: Implementation Handbook. Agency for Healthcare Research and Quality. 2005;4
18. Pollock N, Duong S, Cheng A, et al. Ruling out novel H1N1 influenza virus infection with direct fluorescent antigen testing. Clin Infect Dis. 2009;49:e66–68. [PMC free article] [PubMed]
19. Drexler J, Helmer A, Kirberg H, et al. Poor clinical sensitivity of rapid antigen test for influenza A pandemic (H1N1) 2009 virus. Emerg Infect Dis. 2009;15:1662–1664. [PMC free article] [PubMed]
20. Tang J, Tambyah P, Lai F, et al. Differing symptom patterns in early pandemic vs seasonal influenza infections. Arch Intern Med. 2010;170:861–867. [PubMed]
21. Shiley K, Nadolski G, Mickus T, et al. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol. 2010;31:676–682. [PMC free article] [PubMed]
22. Thompson D, Jungk J, Hancock E, et al. Risk Factors for 2009 Pandemic Influenza A (H1N1)-Related Hospitalization and Death Among Racial/Ethnic Groups in New Mexico. Am J Public Health.2011;101:1776–1784. [PMC free article] [PubMed]
23. Donaldson L, Rutter P, Ellis B, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:b5213. [PMC free article] [PubMed]
24. May L, Lung D, Harter K. An intervention to improve compliance with transmission precautions for influenza in the emergency department: successes and challenges. J Emerg Med. 2010;42:79–85.[PubMed]
25. Vanderweil S, Pelletier A, Hamedani A, et al. Declining antibiotic prescriptions for upper respiratory infections, 1993–2004. Acad Emerg Med. 2007;14:366–369. [PubMed]
26. Gonzales R, Camargo CJ, MacKenzie T, et al. Antibiotic treatment of acute respiratory infections in acute care settings. Acad Emerg Med. 2006;13:288–294. [PubMed]
27. Aspinall S, Good C, Metlay J, et al. Antibiotic prescribing for presumed nonbacterial acute respiratory tract infections. Am J Emerg Med. 2009;27:544–551. [PubMed]
28. Ong S, Nakase J, Moran G, et al. Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction. Ann Emerg Med. 2007;50:213–220. [PubMed]
29. Linder J, Bates D, Platt R. Antivirals and antibiotics for influenza in the United States, 1995–2002. Pharmacoepidemiol Drug Saf. 2005;14:531–536. [PubMed]



