| Author | Affiliation |
|---|---|
| Sean Patrick Nordt, MD, PharmD | University of Southern California, Department of Emergency Medicine, Los Angeles, California |
| Richard F Clark, MD | University of California, San Diego, Department of Emergency Medicine, San Diego, California |
| Edward M Castillo, PhD, MPH | University of California, San Diego, Department of Emergency Medicine, San Diego, California |
| David A Guss, MD | University of California, San Diego, Department of Emergency Medicine, San Diego, California |
ABSTRACT
Introduction:
The treatment of acute coronary syndrome (ACS) includes the administration of aspirin. Current guidelines recommend chewing aspirin tablets to increase absorption. While this is intuitive, there are scant data supporting this recommendation. The purpose of this study is to assess which of 3 different aspirin formulations is most rapidly absorbed after ingestion.
Methods:
A prospective, open-label, 3-way crossover volunteer study at a tertiary university medical center with human subjects 18 years or older. Fasted subjects were randomly assigned to receive aspirin 1,950 mg as (1) solid aspirin tablets swallowed whole, (2) solid aspirin tablet chewed then swallowed, or (3) a chewable aspirin formulation chewed and swallowed. Serum salicylate measurements were obtained over a period of 180 minutes. Pharmacokinetic parameters were determined.
Results:
Thirteen males and 1 female completed all 3 arms of study. Peak serum salicylate concentrations were seen at 180 minutes in all groups. Mean peaks were 10.4, 11.3, and 12.2 mg/dL in groups 1, 2, and 3, respectively. Mean area under the time concentration was 1,153, 1,401, and 1,743 mg-min/dL in groups 1, 2, and 3, respectively. No measurable salicylate concentrations were seen in 6 subjects in group 1 at 60 minutes as compared to 1 subject in group 2. All subjects in group 3 had measurable levels at 45 minutes. There were no adverse effects in any of the subjects during the study period.
Conclusion:
Our data demonstrate that the chewable aspirin formulation achieved the most rapid rate of absorption. In addition, the chewable formulation absorption was more complete than the other formulations at 180 minutes. These data suggest that in the treatment of ACS, a chewable aspirin formulation may be preferable to solid tablet aspirin, either chewed or swallowed.
INTRODUCTION
Current standard practice in the treatment of acute coronary syndrome (ACS) by emergency medicine physicians, internists, and cardiologists, as well as prehospital personnel, includes the rapid administration of aspirin. This is based upon its known antiplatelet effects to decrease clot formation and propagation. The second International Study of Infarct Survival demonstrated a reduction in mortality of 23% when aspirin was administered in acute myocardial infarction.1 These investigators recommended that the first dose of enteric-coated aspirin be either crushed, chewed, or sucked to increase absorption. In clinical practice, patients with suspected ACS are either given chewable aspirin or an intact aspirin tablet to be chewed or swallowed whole. Both the American Heart Association and the American College of Cardiology recommend that aspirin tablets be chewed to increase absorption.2,3 While this is intuitive, there are scant data supporting this recommendation.
Previous studies have compared various aspirin formulations but no 1 study has directly evaluated swallowing solid tablets whole compared to chewing and swallowing a solid tablet or ingesting a chewable tablet formulation.4–6 More recently, both topical and rectal salicylate formulations have also been evaluated.7,8 However, these studies involved in vivo human volunteer subjects, not patients with ACS.
We sought to identify which aspirin formulation is most rapidly absorbed, with a null hypothesis that there is no difference in pharmacokinetic parameters between various formulations of aspirin when administered by the oral route.
METHODS
This was a prospective, open-label, 3-way crossover human volunteer study with subjects acting as their own controls. The study was approved by the university’s human subjects committee and was performed at a tertiary university medical center. Inclusion criteria were as follows: healthy volunteers 18 years of age or older, of either gender or any ethnicity. Subjects could not have used aspirin or other nonsteroidal anti-inflammatory drugs (NSAID) within 7 days of the study. Exclusion criteria included history of aspirin or NSAID hypersensitivity; recent ingestion of anticholinergic, opioid, or other medications affecting gastrointestinal motility; history of peptic ulcer disease or bleeding disorder; history of kidney or liver problems; or pregnancy. Age, weight in kilograms, gender, and ethnicity were recorded. A urine pregnancy test was performed before each arm of the study to ensure female subjects were not pregnant.
On each study day, subjects were directly observed ingesting a total dose of 1,950 mg unbuffered aspirin administered by a study nurse. Subjects were randomly assigned to receive aspirin tablets: group 1 (solid) received six 325-mg aspirin tablets swallowed whole; group 2 (solid-chewed), six 325-mg aspirin tablets chewed then swallowed; and group 3 (chewable), twenty-four 81-mg chewable formulation aspirin tablets that were chewed and swallowed (Kirkland Signature Aspirin 325-mg nonenteric coated solid tablets, Perrigo Company, Allergan, Michigan, or St Joseph’s Chewable Aspirin tablets 81 mg, McNeil Pharmaceuticals, Fort Washington, Pennsylvania). We used a dose of 1,950-mg aspirin after preliminary data showed inability to obtain measurable salicylate concentrations at a dosage of 325 mg. Based on population pharmacokinetic data, for the dose of 1,950 mg, the maximum peak salicylate concentration would be expected to be at the high end of the therapeutic range (5–25 mg/dL).9
There was a washout period of a minimum of 7 days before each arm of the study. Subjects were subsequently randomly assigned before each arm. All doses were administered with a standardized volume of 240 mL of water after fasting for a minimum of 12 hours.
Serum salicylate measurements were obtained at baseline and 5, 10, 15, 20, 30, 45, 60, 120, and 180 minutes after administration of aspirin. Serum salicylate samples were performed at the university medical center chemistry laboratory.
Pharmacokinetic parameters were determined by visual inspection of graph, for example, initial salicylate concentration (C0), time to initial salicylate concentration (Tinit), peak salicylate concentration (Cmax), and time to peak salicylate concentration (Tmax). The area-under-the concentration-time curve was calculated with the trapezoidal method from 0 to 3 hours (AUC3h). Statistical analyses included descriptive statistics of patient demographics and a 2-tailed t-test with correction for continual data, with a P value less than or equal to 0.05 being considered a statistically significant difference. All analyses were performed with SPSS for Windows version 16.0 (SPSS Inc, Chicago, Illinois).
Serum salicylate concentrations were determined with a Beckman Coulter SYNCHRON LX Clinical System (Brea, California). The system uses an immunoassay that catalyzes conversion of salicylate and nicotinamide adenine dinucleotide (reduced) to catechol and nicotinamide adenine dinucleotide in the presence of oxygen. The measurable salicylate concentrations range from 4 to 100 mg/dL.10
RESULTS
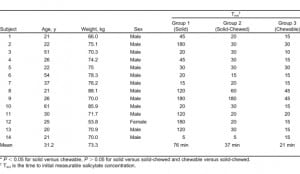
Fourteen subjects (13 male and 1 female) were recruited and all completed all 3 arms of the study. All subjects fasted for a minimum of 12 hours before the study with the exception of water ingestion ad libitum. The mean age of subjects was 31.2 years (20–61 years) with a mean weight of 73.3 kg (53–88 kg). Nine were Caucasian; 4, Asian; and 1, Hispanic. Demographic data and Tinit are presented in the Table. The Cmax was seen at 180 minutes in all groups. The mean Cmax was 10.4, 11.3, and 12.2 mg/dL in groups 1 (solid), 2 (solid-chewed), and 3 (chewable), respectively. There was a statistically significant difference in Cmax between all groups (group 1 [solid] to group 3 [chewable], P = 0.021; group 2 [solid] to group 3 [chewable], P = 0.001) except when comparing solid to solid-chewed (group 1 [solid] to group 2 [solid-chewed], P = 0.28). It should be noted that levels were still increasing at 3 hours and therefore, the true Cmax may have been higher. The mean AUC3h was 1,153, 1,401, and 1,743 mg-min/dL in groups 1 (solid), 2 (solid-chewed), and 3 (chewable), respectively. There was statistically significant difference in AUC3h between all groups (group 1 [solid] to group 3 [chewable], P = 0.002; group 2 [solid-chewed] to group 3 [chewable], P < 0.001) except when solid was compared to solid-chewed (group 1 [solid] to group 2 [solid-chewed], P = 0.166). Of note, 6 of the 14 subjects in group 1 (solid) had no measurable salicylate concentration at 60 minutes, as compared to 1 subject in group 2 (solid-chewed) with no measurable concentration. Conversely, all subjects in group 3 (chewable) had measurable concentrations at 45 minutes. Furthermore, 9 of the subjects in the chewable group had measurable salicylate concentrations at 15 minutes as compared to only 2 in group 1 (solid) and 2 in group 2 (solid-chewed). No subjects reported any adverse effects during the entire course of the study or afterwards. There were no adverse effects in any of the subjects during any of the study periods.
DISCUSSION
Aspirin or acetylsalicylic acid is an acetylated form of salicylic acid with a pKa of 3.5 and a volume of distribution of 0.1 to 0.3 L/kg and is approximately 90% plasma protein bound at therapeutic levels.11 This acetylation is a necessary component for its antiplatelet effects. However, as aspirin is rapidly deacetylated in vivo, salicylic acid concentrations are measured clinically. Aspirin selectively acetylates the hydroxyl group of a serine residue on the prostaglandin H2 synthase enzyme.12 This results in the loss of the cyclooxygenase activity for the life of the platelet.13 Aspirin has been shown in a number of studies to be of benefit in treating patients with ACS.1,14
The treatment of ACS is time-dependent and there are a number of outcome measures, which are routinely evaluated (eg, “door-to-balloon” time for percutaneous transluminal coronary and “door-to-needle” time for thrombolysis). Several studies have suggested improved outcomes with earlier aspirin administration for patients with acute myocardial infarction.15–17
The purpose of this study was to determine which formulation of aspirin tablets achieves the most rapid rate of absorption. Our data demonstrate that administering a chewable aspirin formulation achieved more rapid absorption at the doses we administered. We also identified that solid aspirin tablets when swallowed whole had a more variable rate of absorption compared to the chewable formulation, which showed not only more rapid but also more complete absorption.
Our study is the first study to our knowledge directly comparing unbuffered solid aspirin tablets, either chewed or swallowed whole, to a chewable formulation of aspirin.
A 3-armed crossover study was previously performed in human volunteers receiving either buffered aspirin 325 mg as a solid tablet, chewed or swallowed whole, compared to an Alka-Seltzer solution (Bayer, Morristown, New Jersey) containing 325 mg aspirin.4 These authors did not have a chewable aspirin arm of the study. The maximum salicylate concentrations were seen at 1 hour in both the chewable and Alka-Seltzer arms compared to 2 hours when tablets were swallowed whole. In addition, the peak salicylate concentrations were higher in the chewable arm compared to the other 2 arms. The maximal beneficial antithrombotic effect of aspirin is seen when platelet inhibition exceeds 90% of baseline.4 Ninety percent platelet inhibition was seen at between 13 to 14 minutes after chewing an aspirin tablet as compared to 19 to 20 minutes and 25 to 26 minutes after Alka-Seltzer and whole tablet ingestion, respectively.
One general limitation in extrapolating from the above study is the use of buffered tablets and Alka-Seltzer, as many institutions and prehospital providers use unbuffered aspirin tablets. Buffering agents (eg, magnesium oxide, calcium carbonate) are added to minimize gastric upset by creating increased local pH, thereby limiting absorption in the stomach. Alka-Seltzer contains sodium bicarbonate for similar reasons. By causing a higher intraluminal pH, and subsequent increase in fraction of ionized salicylate, these buffering agents may delay absorption. Unbuffered aspirin results in a lower gastric pH and increased absorption from the stomach than does buffered aspirin.18 We suggest only unbuffered aspirin be used in patients with ACS to facilitate more rapid and complete absorption.
In another crossover volunteer study, subjects were given a 325-mg aspirin tablet swallowed whole, four 81-mg chewable aspirin tablets, or an Alka-Seltzer solution containing 325-mg aspirin.5 Peak salicylate concentrations were seen at 40 minutes, the endpoint of their study, in all groups. However, both the chewable and the Alka-Seltzer solution had higher peak concentrations than aspirin tablet swallowed whole. Interestingly, the time to greater than 90% platelet inhibition was seen within 10 minutes in the chewable arm compared to 15 minutes and 25 minutes in the Alka-Seltzer and solid tablet arms, respectively. These data appear to support using a chewable aspirin formulation.
In another triple crossover study, volunteers were administered one 81-mg, two 81-mg, or four 81-mg chewable aspirin tablets and the time and degree of platelet inhibition was measured.6 For all subjects in all 3 arms, greater than 90% platelet inhibition was seen at 30 minutes. However, in the initial phase of their study, 3 subjects receiving 81 mg and 1 subject given 162 mg did not have platelet inhibition at 15 minutes, whereas all subjects receiving 324 mg achieved platelet inhibition at 15 minutes. These results suggest it may be preferable to give a higher dose of aspirin, particularly to patients with ACS.
We theorize that a more rapid absorption was observed in group 3 (chewable) after ingesting the chewable formulation aspirin, for several reasons. First, in order for a drug to be absorbed it must first be solubilized. Chewable formulations are softer tablets that dissolve more quickly than hard-pressed tablets meant for swallowing intact. Furthermore, giving twenty-four 81-mg chewable tablets compared to six 325-mg solid tablets would result in an increase in total amount of both gastric and small intestinal surface area coverage. A larger amount of surface area in contact with aspirin would be expected to increase the amount of aspirin absorbed. Giving multiple chewable tablets, that is, four 81-mg tablets compared to just one 325-mg solid aspirin tablet, should mimic this surface area effect. This should increase rapidity and completeness of platelet inhibition, the desired endpoint in the treatment of ACS.
Higher-dose administration has been associated with an increased incidence of bleeding in persons chronically ingesting aspirin.19 A more recent study using combined GUSTO I and III data in patients with ST-elevation myocardial infarction suggests a possible increase in bleeding complications with 325-mg aspirin compared to 162 mg.20
There is also a concern of increased gastrointestinal upset at higher dosages. Of note, none of our subjects had any adverse events, including gastrointestinal upset, after ingesting 1,950 mg on 3 separate occasions during or after the study period.
In patients with suspected ACS, the use of two 81-mg (162 mg) and possibly four 81-mg (324 mg) chewable tablets may be preferable to increase intraluminal surface area to aspirin and subsequent absorption and platelet inhibition.
LIMITATIONS
The major limitation of our study is that we did not measure platelet inhibition activity but rather serum salicylate concentrations, as this was a pharmacokinetic study. However, aspirin is well known to inhibit platelet aggregation. Previous data have identified that a serum salicylate concentration of approximately 1 mg/dL is necessary to achieve platelet inhibition greater than 90% of baseline.4 Similar to other studies, the lowest level of detection for the assay we used to determine serum salicylate concentrations was 4 mg/dL.8 Therefore, all subjects would have been expected to have “full” antiplatelet effects by the time we achieved measurable concentrations. However, the delay in Tinit, particularly in group 1 (solid) after swallowing an aspirin tablet whole, suggests this effect would be achieved later in group 1 (solid) compared to group 2 (solid-chewed) and group 3 (chewable).
As stated previously, we used supratherapeutic doses of 1,950-mg aspirin, on the basis of preliminary data we obtained after the administration of 325-mg tablets to 3 subjects, without the ability to obtain measurable salicylate concentrations owing to the sensitivity of our assay. In addition, although salicylate does exhibit zero-order pharmacokinetics at extremely high dosages (ie, large poisonings), at the doses we used, salicylate follows first-order kinetics.21,22 One further limitation of study is that our subject population lacked some ethnic and gender diversity. However, it is unclear how this affects our results.
Finally, as this was a pharmacokinetic study, we used fasting subjects. It is possible in real-world patients with “full stomachs” that absorption could be further delayed owing to decreased gastric emptying time.
CONCLUSION
Our data demonstrate that the chewable formulation of aspirin, as well as the chewing of solid tablets, increases the rate of absorption. These pharmacokinetic data suggest that a chewable formulation may be preferable to solid tablets chewed or swallowed in treatment of ACS. However, larger prospective studies in patients being treated for ACS are needed to see if this is clinically significant, ideally also assessing the degree of platelet inhibition.
Footnotes
This study was supported in part by an AHA SEED GRANT AWARD.
Supervising Section Editor: Jeffrey R. Suchard, MD
Submission history: Submitted February 5, 2011; Revision received April 20, 2011; Accepted April 25, 2011
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.4.2222
Address for Correspondence: Sean Patrick Nordt, MD, PharmD
University of Southern California, Department of Emergency Medicine, LACþUSC Medical Center, 1200 N State St, Rm 1011,Los Angeles, CA 90033
E-mail: spnordt@hotmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;;13:349–360.
2. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care, part 7: the era of reperfusion, section 1—acute coronary syndromes (acute myocardial infarction) Circulation. 2000;;102:I172–I203.
3. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction. J Am Coll Cardiol.2007;;50:1–157.
4. Feldman M, Cryer B. Aspirin absorption rates and platelet inhibition times with 325-mg buffered aspirin tablets (chewed or swallowed intact) and with buffered aspirin solution. Am J Cardiol.1999;;84:404–409. [PubMed]
5. Schwertner HA, McGlasson D, Christopher M, et al. Effects of different aspirin formulations on platelet aggregation times and on plasma salicylate concentrations. Thromb Res. 2006;;118:529–534. [PubMed]
6. Dabaghi SF, Kamat SG, Payne J, et al. Effects of low-dose aspirin on in vitro platelet aggregation in the early minutes after ingestion in normal subjects. Am J Cardiol. 1994;;74:720–723. [PubMed]
7. Maalouf R, Mosley M, Kallail J, et al. A comparison of salicylic acid levels in normal subjects after rectal versus oral dosing. Acad Emerg Med. 2009;;16:157–161. [PubMed]
8. Tanen DA, Danish DC, Reardon JM, et al. Comparison of oral aspirin versus topical applied methyl salicylate for platelet inhibition. Ann Pharmacother. 2008;;42:1396–1401. [PubMed]
9. McAuley DF. The clinicians ultimate reference. GlobalRPh Web site. Available at:http://www.globalrph.com/labs_drug_levels.htm. Accessed April 18, 2011.
10. Beckman Coulter. SYNCHRON System(s) SALY Chemistry Information Sheet Salicylate. Beckman Coulter, Inc Web site. Available at:http://www.beckmancoulter.com/customersupport/IFU/cis/A18551/AE/EN_SALY.pdf . Accessed December 30, 2009.
11. Needs CJ, Brooks PM. Clinical pharmacokinetics of the salicylates. Clin Pharmacokinet.1985;;10:164–177. [PubMed]
12. Schrör K. Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemostas. 1997;;23:349–356.
13. Patrono C, Ciabattoni G, Patrignani P, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985;;72:1177–1184. [PubMed]
14. Lewis HD, Davis JW, Archibold DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina: results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;;309:396–403. [PubMed]
15. Barbash IM, Freimark D, Gottlieb S, et al. Outcome of myocardial infarction in patients with aspirin is enhanced by pre-hospital administration. Cardiology. 2009;;98:141–147. [PubMed]
16. Abdelnoor M, Landmark K. Infarct size is reduced and the frequency of non-Q-wave myocardial infarction is increased in patients using aspirin at the onset of symptoms. Cardiology. 1999;;91:119–126. [PubMed]
17. Eisenberg MJ, Topal EJ. Prehospital administration of aspirin in patients with unstable angina and acute myocardial infarction. Arch Intern Med. 1996;;156:1506–1510. [PubMed]
18. Dotevall G, Ekenved G. The absorption of acetylsalicylic acid from the stomach in relation to intragastric pH. Scand J Gastroenterol. 1976;;11:801–805. [PubMed]
19. Slattery J, Warlow CP, Shorrock CJ, et al. Risks of gastrointestinal bleeding during secondary prevention of vascular events with aspirin: analysis of gastrointestinal bleeding during the UK-TIA trial. Gut. 1995;;37:509–511. [PMC free article] [PubMed]
20. Berger JS, Stebbins A, Granger CB, et al. Initial aspirin dose and outcome among ST-elevation myocardial infarction patients treated with fibrinolytic therapy. Circulation. 2008;;117:192–199.[PubMed]
21. O’Malley GF. Emergency department management of the salicylate-poisoned patient. Emerg Med Clin North Am. 2007;;25:333–346. [PubMed]
22. Flomenbaum NE. Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s Toxicologic Emergencies. 8th ed. New York, NY: McGraw-Hill;; 2006. Salicylates; pp. 550–564.


