| Author | Affiliation |
|---|---|
| Mark A. Saks, MD, MPH | Drexel University College of Medicine, Department of Emergency Medicine, Philadelphia, PA |
| Sharon Griswold-Theodorson, MD | Drexel University College of Medicine, Department of Emergency Medicine, Philadelphia, PA |
| Furkan Shinaishin, MD | INOVA Alexandria Hospital, Alexandria, VA |
| Dawn Demangone, MD | Virtua West Jersey Hospital, Voorhees, NJ |
ABSTRACT
This case report describes a patient with a subacute right-sided tension hemopneumothorax following an occult stab. The patient’s electrocardiogram (ECG), performed as part of a standardized triage process, demonstrated significant abnormalities that misguided initial resuscitation, but resolved following evacuation of the tension hemopneumothorax. Tension pneumothorax is typically regarded as an immediately life-threatening condition that requires emergent management with needle or tube thoracostomy. However, we believe that subacute tension pneumothorax may be a rarely observed clinical phenomenon and may lead to unique ECG findings. We believe that the ECG changes we observed provided an early clue to the eventual diagnosis of a subacute tension pneumothorax and have not been previously described in this setting.
INTRODUCTION
Tension pneumothorax is traditionally regarded as an immediately life-threatening condition that requires emergent management with needle or tube thoracostomy to prevent hemodynamic collapse. However, a number of recent reports have challenged this dogma.1–4 Instead of a homogeneous disease state, there is likely a subset of clinically well-appearing, hemodynamically stable patients in whom respiratory failure progresses much more slowly due to their ability to compensate through other mechanisms.5 Therefore, many, if not all, of the classic symptoms may be absent, leaving only non-specific findings such as tachycardia and tachypnea. In these patients, a confirmatory study, such as a chest x-ray, echocardiogram or thoracic ultrasound, may be indicated to rule out other causes of chest pain or shortness of breath, confirm the diagnosis, and prevent unnecessary intervention with invasive procedures.
While a subacute tension pneumothorax should be readily seen on chest x-ray, electrocardiogram (ECG) changes associated with pneumothorax are well described and may be helpful in triggering recognition of the diagnosis in the event of a delay in obtaining x-ray. Initial studies were conducted in the 1920s when artificial pneumothorax therapy was used in the treatment of tuberculosis.6–11More recently, numerous case reports of ECG changes in the setting of predominantly left-sided spontaneous pneumothorax and tension pneumothorax have also been published. From these reports, a typical pattern of reversible ECG changes in the presence of left-sided pneumothorax has emerged: a rightward frontal QRS axis shift with diminution of precordial R voltage, decreased QRS amplitude, and T wave inversions in the precordial leads.12,13 However, ECG changes due to right-sided pneumothorax, tension pneumothorax, or hemothorax are largely non-specific and are not as well described as those with left-sided findings.14–16 In addition, to our knowledge, reversible changes have not been previously noted in the literature. This may be because they demonstrate less consistent findings on ECG, have less pronounced ECG changes, or because right-sided injury occurs less frequently.6,17–19
CASE REPORT
A 46-year-old male with a past medical history only of treated tuberculosis presented to our emergency department (ED) via ambulance after being found lying in the hallway of his apartment building. The paramedics did not find signs of trauma at the scene. The patient was fully alert and oriented and complaining of right-sided upper abdominal and chest pain. He was afebrile with a blood pressure of 92/65mm Hg, heart rate 89 beats/min, respiratory rate 24 breaths/min, and an O2 saturation of 96% on room air. On repeat measurement, blood pressure and respiratory rate improved spontaneously and remained stable throughout the remainder of his ED course. On questioning, the patient was uncertain of the duration of his pain but stated that he believed it began upon awakening earlier in the morning. He denied associated nausea, vomiting, dyspnea, fevers, chills, cough, recent illness, or similar past episodes and was able to tolerate oral food and fluids without difficulty prior to arrival. He denied taking any medications and had no known drug allergies. His social history was positive for alcohol and crack cocaine use, most recently on the night prior to arrival.
Physical examination revealed a thin, middle-aged male with bi-temporal wasting who was speaking easily and did not appear to be in any distress. He had no obvious signs of recent trauma. Of note, there was no blood visible on examination of his skin, clothing, or stretcher. He had no jugular venous distension, his trachea was midline, and his cardiovascular examination was normal. However, the lung examination revealed decreased breath sounds on the right and diffuse abdominal tenderness with voluntary guarding that was increased in the mid-epigastrium and right upper quadrant. Peritoneal signs were absent, and the patient tested heme-occult negative on rectal examination.
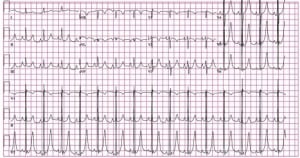
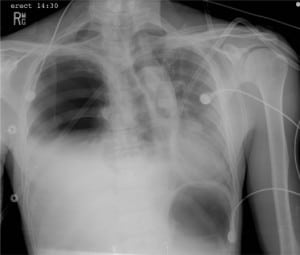
An initial ECG obtained at triage revealed a normal sinus rhythm at 91 beats/min with a QRS axis of +75°and a QT interval (QTc) of 450ms. A large QRS voltage, prominent peaked T waves in the lateral pre-cordial leads, and prominent P waves inferiorly were also noted (Figure 1). Treatment at triage was initiated with oxygen, aspirin, and nitrates. Following physician evaluation, intravenous calcium was administered despite no known history of renal insufficiency or hyperkalemia and the patient was given morphine 2mg IV with complete resolution of his pain. Following these early interventions, a repeated ECG showed no change. A portable chest x-ray was then obtained, which revealed a right-sided tension hemopneumothorax (Figure 2).
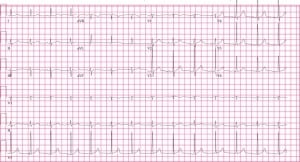
Additional physical examination at that time revealed a healing 1cm linear laceration in the right posterior axillary skin fold. No crepitus, erythema, bleeding, or dried blood was present at or surrounding the site. When confronted, the patient admitted to a stab wound with an unknown object three days earlier. Thoracostomy was performed with immediate return of 1200cc of dark, non-clotted blood, and he was admitted to the hospital by the trauma service. Repeat ECG following thoracostomy revealed resolution of the peaked T waves, changes in the voltage of the P and QRS waves, a shortening of the QTc, and a shift in the QRS axis (Figure 3). The patient’s initial serum potassium was 3.9 mmol/L (normal 3.5–5.0 mmol/L), and he did not develop any abnormalities in serum cardiac markers during his hospitalization. Cardiac ultrasonography was not performed. The chest tube was removed on the second day without re-accumulation of the hemopneumothorax and the patient was discharged on hospital day three.
DISCUSSION
At presentation, our patient was hemodynamically stable, without respiratory distress, complaining of chest and abdominal pain and, therefore, had an ECG performed as per triage protocols leading to a delay in the diagnosis of his subacute tension pneumothorax. One reason for this is that the initial ECG exhibited many abnormalities that we believe are a result of his underlying pathology. Among the most impressive changes noted were the prominent T waves and prolonged QTc. Hyper-acute T waves may be a normal variant or secondary to early repolarization, but are also commonly seen in early myocardial ischemia, left ventricular hypertrophy (LVH), and hyperkalemia.20 Therefore, our initial therapy was aimed at resolving these common conditions: nitrates, aspirin and oxygen for cardiac ischemia and calcium for hyperkalemia. An interval ECG following these interventions showed no significant change in T wave morphology, although they did resolve following chest tube placement. Prior reports of T-wave changes in the setting of a right-sided pneumothorax or tension pneumothroax have revealed no change, decreased amplitude, or inversion type-changes.6,8,11,17However, prominent T waves have not been previously demonstrated.
While a prolongation in the QTc may be due to a congenital syndrome, it is also seen in CNS system disease, metabolic syndromes (hypokalemia, hypocalcemia, hypothyroidism, hypothermia), mitral valve prolapse, or with numerous classes of pharmaceutical agents.20 This finding has also been reported in the setting of pneumothorax, although the degree of QT prolongation does not necessarily correlate with the size.21 Of note, our patient’s initial corrected QTc, as measured by Bazzett’s Formula, was 450ms, which was decreased to 420ms following evacuation of the tension pneumothorax.
Several possible mechanisms have been proposed to account for the presence of reversible ECG changes in patients with a right-sided pneumothorax. First, the pneumothorax may induce a change in the anatomic position of the heart within the thorax. A clockwise rotation of the heart around its long axis when viewed from the apex has been described in association with right-sided pneumothorax.6–8,10,11,22 Therefore, the anterior portion of the right ventricle rotates to the left and becomes more anterior, closer to the left chest wall than the left ventricle. This changes the position of the right ventricle relative to the ECG leads and, therefore, influences findings on the ECG. Additionally, a change in the intra-thoracic air mass may manipulate the ECG tracing by shifting the heart within the thorax.6,11,22–25 Phasic voltage variations or electrical alternans that resolve following pneumothorax evacuation have been described, suggesting that the position of the heart in the thorax may shift from beat-to-beat or with respiration.26,27 Our patient’s initial chest x-ray (Figure 2) reveals a lateral shift of the heart within the thorax associated with rotation of the mediastinal structures consistent with the right, anterior, oblique position. Following placement of the chest thoracostomy tube, the cardiac and mediastinal structures revert to their normal anatomic position as seen on repeat chest x-ray. Interestingly, however, our patient did not exhibit a left axis deviation on the initial ECG as might be expected with this anatomic shift, nor did he did have a relative rightward axis deviation following evacuation.
Another potential influence on the ECG may be an elevated pulmonary resistance created by the pneumothorax, leading to acute right ventricular strain and the demonstration of findings typically seen with cor pulmonale.8,11,18,22,28,29 Our patient’s initial ECG demonstrated a normal axis and prominent P waves in the inferior leads, but did not reveal other findings typically seen in this setting. Interestingly, the literature has not demonstrated a correlation between the size of a right-sided pneumothorax and degree of changes apparent on the ECG.
Finally, a disruption of the equilibrium maintained between the influences of the right and left sympathetic nerves may result in an abnormal repolarization pattern and, therefore, a prolonged QTc.26 In an animal model, a decrease in the relative contribution of the right stellate ganglia has been shown to influence mycocardial repolarization, leading to a prolongation of the QTc with simultaneous increases in T wave amplitude.30 Perhaps the right-sided tension pneumothorax in our patient exerted a similar, negative influence on the right stellate ganglion, resulting in the prominent T waves and prolonged QTcs that resolved after evacuation equalized the influences of the right and left stellate ganglion.
In sum, there is likely a subset of clinically well-appearing, hemodynamically stable patients with tension pneumothorax in whom respiratory failure progresses much more slowly. In these patients, an ECG, often obtained during the routine triage process, may suggest this diagnosis and can then be confirmed with additional studies such as a chest x-ray, or thoracic ultrasound to avoid unnecessary procedures. We reported several reversible ECG findings, likely influenced by a combination of factors directly related to the tension hemopneumothorax, which may also aid in making this challenging diagnosis.
Footnotes
Supervising Section Editor: Eric R. Snoey, MD
Submission history: Submitted August 17, 2009; Revision Received November 23, 2009; Accepted December 7, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Mark Saks MD, MPH Department of Emergency Medicine Drexel University College of Medicine Mail Stop 1011 245 N. 15th St. Philadelphia PA 19102-1192
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Wa Chan SS. Tension Pneumothorax Managed without Immediate Needle Decompression. J Emerg Med. 2009;36:242–45. [PubMed]
2. Boon D, Llewellun T, Rushton P. A Strange Case of a Tension Pneumothorax. Emerg Med J.2002;19:470–1. [PMC free article] [PubMed]
3. Watts BL, Howell MA. Tension pneumothorax: a difficult diagnosis. Emerg Med J. 2001;18:319–20. [PMC free article] [PubMed]
4. Holloway VJ, Harris JK. Spontaneous pneumothorax: is it Under Tension? J Accid Emerg Med.2000;17:222–3. [PMC free article] [PubMed]
5. Leigh-Smith S, Harris T. Tension Pneumothorax- time for a rethink? Emerg Med J. 2005;22:228–16.
6. Armen RN, Frank TV. Electrocardiographic Patterns in Pneumothorax. Dis . Chest. 1949;15:709–19. [PubMed]
7. Hansen OS, King FW. The influence of pulmonary collapse on the electrocardiogram. Am Rev Tuberc. 1930;22:320–336.
8. Master AM. The electrocardiographic changes in pneumothorax in which the heart has been rotated. Am Heart J. 1928;3:472–83.
9. Weinshel M, Mack I, Gordon A, et al. Electrocardiographic changes accompanying pulmonary collapse therapy and thoracic surgery. Am Rev Tuberc. 1951;64:50–63. [PubMed]
10. Anderson AR. Electrocardiographic studies in artificial pneumothorax and thoracoplasty. Am Rev Tuberc. 1929;20:728–35.
11. Feldman D, Silverberg C. Electrocardiographic changes in pulmonary collapse therapy. Circ.1950;1:982–90.
12. Walston A, Brewer DL, Kitchens CS, et al. The electrocardiographic manifestations of spontaneous left pneumothorax. Ann Int Med. 1974;80:375–379. [PubMed]
13. Strizik B, Forman R. New ECG changes associated with a tension pneumothorax. Chest.1999;115:1742–44. [PubMed]
14. Quinn MW, Dillard TA. Delayed traumatic hemothorax on ticlopidine and aspirin for coronary stent. Chest. 1999;116:257–60. [PubMed]
15. Gregory WT, Patton PE. Isolated pleural effusion in severe ovarian hyperstimulation: a case report. Am J Ob & Gyn. 1999;180:1468–1471.
16. Pronchik DJ, Sexton J. Emergency department presentation of an unusual pleural effusion. Am J Emerg Med. 1998;16:163–5. [PubMed]
17. Alkihan M, Biddison JH. Electrocardiographic changes with right-sided pneumothorax. South Med J. 1998;91:677–80. [PubMed]
18. Diamond JR, Estes NM. ECG changes associated with iatrogenic left pneumothorax simulating anterior myocardial infarction. Am Heart J. 1982;103:303–305. [PubMed]
19. Habibzadeh M. ECG changes associated with spontaneous left-sided pneumothorax. Postgraduate Med. 1980;68:221–224.
20. Chan TC, Brady WJ, Harrigan RA, et al., editors. ECG in Emergency Medicine and Acute Care.Philadelphia, PA: Elsevier-Mosby; 2005.
21. Athanasopoulos C, Childers R. Q-T prolongation in acute pneumothorax. Acta Cardiologica.1979;2:85–93. [PubMed]
22. Paige GB, Spalding K. Electrocardiographic changes as the first indicator of a right pneumothorax in an anesthetized child. source>Anesthesiology. 1996;85:1200-02.
23. Kuritzky P, Goldfarb AL. Unusual electrocardiographic changes in spontaneous pneumothorax.Chest. 1976;70:535–537. [PubMed]
24. Feldman T, January CT. ECG changes in pneumothorax: a unique finding and proposed mechanism. Chest. 1984;86:143–5. [PubMed]
25. Kamimura M, Kudo K. ECG changes in tension pneumothorax: a hypothesis. Chest.2000;117:1527. [PubMed]
26. Kounis NG, Aavras GM, Kitrou MP, et al. Unusual electrocardiographic manifestations in conditions with increased intrathoracic pressure. Acta Cardiologica. 1988;43:653–61. [PubMed]
27. Kozilj M, Rakovec P, Sok M. Unusual ECG variations in left-sided pneumothorax. J Electrocard.1997;30:109–111.
28. Summers RS. The electrocardiogram as a diagnostic aid in pneumothorax. Chest. 1973;63:127–28. [PubMed]
29. Bronfin ID, Saling S, Black LT. Electrocardiographic studies in artificial pneumothorax – a report on 110 cases. Tubercle. 1929:114–23.
30. Rogers M, Abildskow J, Preston J. Cardiac effects of Stimulation and Block of the Stellate Ganglion. Anesthesiology. 1973;39:525–33. [PubMed]


