| Author | Affiliation |
|---|---|
| Michael D. Michel, MD | Department of Emergency Medicine, Indiana University, Indianapolis, Indiana |
| Louise W. Kao, MD | Department of Emergency Medicine, Indiana University, Indianapolis, Indiana |
| Brian K. Sloan, MD | Department of Emergency Medicine, Indiana University, Indianapolis, Indiana |
INTRODUCTION
Primary meningococcal arthritis (PMA) is a rare infectious disease that occurs in as little as 1% of meningococcal infections.1 PMA is arthritis without meningitis, fever, rash, and hemodynamic instability.2 It is usually preceded by an upper respiratory infection in 50–55% of presentations, and patients may appear nontoxic, afebrile, and polyarthralgic. Despite definition they may have a rash.3–12
Although PMA is rare, one would expect immunocompromised patients to be more susceptible to develop this disease. However, in areas of high prevalence of human immunodeficiency virus (HIV) infection, just outside the meningococcal belt in the Gabonese Republic of Africa, HIV-infected patients were not found to be at higher risk for Neisseria meningococcal disease.13 Furthermore, a small study by Skeete et al14 showed patients with a compromised immune system were not more likely to have normal peripheral white blood cell (WBC) count during septic arthritis.
With such a variable presentation of septic arthritis, it can be extremely difficult to diagnose a septic joint without a high clinical suspicion. Numerous studies indicate that previous joint disease and low socioeconomic class do appear to be risk factors for developing septic arthritis, although N. meningococcal disease was not included.12 There are no well-studied or validated clinical criteria that can be used when attempting to diagnose PMA. While the literature varies most orthopedists will use a WBC count of 5.0–10.0 × 103 cells/μL or greater and no synovial fluid crystals before they will consider the diagnosis of a septic joint. Septic arthritis has been diagnosed by intra-articular cultures in 7–10% of cases with a joint fluid aspirate WBC count of less than 10.0 × 103 cells/μL, but none of these studies included N. meningococcal disease. The literature also supports the finding that traditional septic arthritis joints have an elevation in at least 1, if not several, of the following: peripheral WBC count, erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), or joint aspirate WBC.15–17 Furthermore, when looking at the case studies published, we found that all but 4 cases of PMA had purulent aspirate, with these 4 cases having synovial WBC count ranging from 17–163,000 cells/μL.6–8,18 We will present a unique case of PMA with non-purulent joint aspirate with normal peripheral WBC and joint fluid WBC count of 8.7 × 103 cells/μL in an HIV-positive patient with a CD4 count reflecting a functional immune system.
CASE PRESENTATION
A homeless, 60-year-old man with a history of HIV presented to the emergency department (ED) with chief complaint of bilateral wrist pain and left ankle swelling and pain for a day. Patient stated pain was 10/10, constant, increased with movement and was not relieved by ibuprofen. He stated that he had a history of gout attack and this presentation was similar to previous gouty attacks. He denied intravenous drug abuse, fever, or recent illness. In regards to his HIV status, the patient had a CD4 count of 604 and an HIV RNA PCR quantity of 120,000 1 month prior to presentation. On physical examination his vital signs were positive for a low grade fever of 37.5 °C and tachycardia of 120 beats per minute (bpm). He appeared non-toxic and was without rashes, but showed limitation of active movement of bilateral wrists and left ankle due to pain with warmth and tenderness to palpation in all 3 joints. He had trace edema of the left ankle. No labs were drawn on the initial visit, and he was treated with 1.2 mg colchicine, followed by 4 subsequent doses of 0.6 mg, 800 mg ibuprofen, and 5/325 mg tab of hydrocodone/acetaminophen with significant decrease in his pain and ability to move all joints and bear weight. He was subsequently discharged home with a prescription for ibuprofen and hydrocodone/acetaminophen with diagnosis of HIV and acute gout attack.
The patient returned 3 days after discharge with worsening pain, erythema, and swelling in his left ankle and new right ankle and left knee pain, erythema, and edema. His vitals were normal without a low grade fever, but tachycardia of 122 bpm. The patient remained non-toxic appearing without a rash. Labs were obtained, arthrocentesis of his left knee was performed, and he was treated with ketorolac for pain with improvement. His laboratory analysis showed a peripheral WBC of 9.4, uric acid of 6.9 mg/dL, and joint aspirate WBC count of 8.7 × 103 cells/μL without crystals. He was discharged with nonsteroidal anti-inflammatory drugs, rheumatology follow up and diagnosis of inflammatory arthritis.
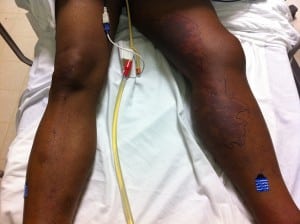
Two days after being discharged the second time, the patient was found sitting on a bench across from the homeless shelter unresponsive. He had been there all night. Emergency medical services were called and he was given naloxone without response. On presentation to the ED he was unresponsive with vitals showing hypertension of 151/84, temperature of 36.3°C and tachycardia of 138 bpm. On physical examination he was noted to have entire left lower extremity edema with purpura noted throughout left leg and right ankle (Figure). A computed tomography (CT) of his left leg showed edema only, and a head CT was negative. His labs showed acute renal failure with Cr of 2.2 and a peripheral WBC of 6.6 with 17% bands. His knee cultures from the previous ED visit were growing gram negative coccobacilli. The orthopedic service was consulted and took the patient to the operating room (OR) for washout.
Post-operatively he was admitted to the intensive care unit where he was subsequently intubated for airway protection due to declining mental status. His blood and cerebral spinal fluid cultures grew N. meningitides, and he required xigris and levophed for septic shock. He had a prolonged hospital course that included multiple surgical procedures for debridement/washouts, I&Ds of bilateral lower extremities due to infected wounds from purpura fulminans (requiring wound vac management), and skin grafts to the left lower extremity. He had another episode of acute renal failure due to urinary retention, causing hydronephrosis that required a urology consult without causal finding, and he was taught to perform in-and-out catheterizations.
The patient was diagnosed with mild syndrome of inappropriate antidiuretic hormone and placed on fluid restriction. He experienced left-sided hearing loss due to meningitis After developing a right upper extremity deep vein thrombosis he was bridged from enoxaparin to warfarin for treatment. He also had to be treated for hospital-acquired urinary tract infections, required multiple blood transfusions due to the operations, and was placed on nutritional supplementation for malnourishment.
It should be noted that neurology was consulted due to prolonged altered mental status after extubation. The patient had a negative magnetic resonance image of the brain and electroencephalography. Infectious disease was also consulted and treated the initial infection with a 14-day course of ceftriaxone and the lower extremity wound infections with a course of vancomycin and meropenem. After 62 days of hospitalization, he was discharged to a sub-acute rehabilitation facility for further management.
DISCUSSION
This case presentation represents the only known case of a patient with primary meningococcal arthritis with nonpurulent joint aspirate with a WBC count of less than 10.0 × 103 cells/μL and a normal peripheral WBC count. While the literature suggests that in septic arthritis the patient can present appearing clinically nontoxic and afebrile with normal labs and a history of previous joint disease, our case represents the perfect storm for missing an infected joint with terrible morbidity resulting to the patient.
On the initial presentation, the differential diagnosis included septic arthritis. However, due to the alleged history of previous gout attack similar to the initial described presentation it was decided to treat the patient for acute gout and if there was no improvement to draw labs and perform an arthrocentesis. Unfortunately for the patient, he improved with gouty management, was able to ambulate, and asked to be discharged from the ED. The decision to not perform arthrocentesis is one that has been discussed at this author’s morbidity and mortality conference and was mostly due to playing the odds.
When the patient re-presented to the ED, arthrocentesis was performed, as were peripheral labs; however, peripheral WBC and joint aspirate WBC were well below the reported values that constitute a septic joint in our institution. Given the labs and the nontoxic appearance of the patient he was treated symptomatically and referred to outpatient rheumatology for further evaluation of possible inflammatory arthritis. Fortunately for the patient, cultures of the knee aspirate were obtained prior to discharge, which helped the admitting team when he presented septic. The decision to discharge with urgent rheumatology follow up was discussed, in addition to having orthopedic surgery evaluate the patient in the ED. However, the circumstances were such that the consulting team would have had little reason to admit, much less take the patient to the OR: he was nontoxic, afebrile, and joint aspirate was non-purulent with less than a WBC count of 10.0 × 103 cell/μL. One could argue that quantitative inflammatory markers (ESR and CRP) should have been obtained; however, these results infrequently change management in acute septic arthritis.19 Additionally, if these markers were hypothetically elevated the next step would have been arthrocentesis. In this case the clinicians elected to skip this intermediate step and proceed to arthrocentesis.
CONCLUSION
We present this case to raise awareness among physicians of the atypical presentations of septic arthritis. More specifically, there are patients with a confirmed diagnosis of septic arthritis even though the synovial fluid WBC is lower than most reported guidelines. Methicillin-resistantStaphylococcus aureus and Neisseria are the 2 bacterial infections that have traditionally low synovial fluid WBC, although as stated earlier there has not been a report of Neisseria in a patient with a synovial WBC count of less than 10.0 × 103 cells/μL.
Footnotes
Supervising Section Editor: Rick A. McPheeters, DO
Submission history: Submitted July 1, 2012; Revisions received October 5, 2012; Accepted October 26, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.10.12906
Address for Correspondence: Michael D. Michel, MD, Department of Emergency Medicine, Indiana University, 1701 N. Senate Blvd, B401, Indianapolis, IN 46202. Email: mimichel@iupui.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Vienne P, Ducos-Galand M, Guiyoule A, et al. The Role of Particular Strains of Neisseria meningitides in Meningococcal Arthritis, Pericarditis, and Pneumonia. Clin Infect Dis.2003;37(15):1639–1642. [PubMed]
2. Harwood M, Womack J, Kapur R. Primary Meningococcal Arthritis. JABFM. 2008;21(1):66–69.[PubMed]
3. Schaad U. Arthritis in disease due to Neisseria meningitides. Rev Infect Dis. 1980;2(6):880–888.[PubMed]
4. Salmeron C, Mart M, Richet H, et al. Primary meningococcal polyarthritis. J Infect.1986;13(3):281–283. [PubMed]
5. Bilavsky E, Yarden-Bilavsky H, Zevit N, et al. Primary meningococcal arthritis in a child: Case report and literature review. Scand J Infect Dis. 2006;38(5):396–399. [PubMed]
6. McCulloch M, Brooks H, Kalantarinia K. Isolated Polyarticular Septic Arthritis: An Atypical Presentation of Meningococcal Infection. Am J med Sci. 2008;335(4):323–326. [PubMed]
7. Bhavnagri S, Steele N, Massasso D, et al. Meningococcal-associated arthritis: infection versus immune-mediated. Intern Med J. 2008;38(1):71–73. [PubMed]
8. Davis B, Pasternack M. Case 19-2007: A 19-Year-Old College Student with Fever and Joint Pain.NEJM. 2007;356(25):2631–2637. [PubMed]
9. McMullen B. An Infant with meningococcal arthritis of the hip. J Paediatr Child Health.2009;45(12):762–763. [PubMed]
10. Rathore M. Meningococcal Arthritis: Comparison of Children and Adults. Infect Dis Clin Pract.1993;2(4):282–287.
11. Mathews C, Weston VC, Jones A, et al. Bacterial septic arthritis in adults. The Lancet.2010;375(9717):846–855. [PubMed]
12. Gupta M, Sturrock R, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24–30. [PubMed]
13. Nkoumou M, Betha G, Kombila M, et al. Bacterial and Mycobacterial Meningitis in HIV-Positive Compared with HIV-Negative Patients in an Internal Medicine Ward in Libreville, Gabon. J Acquir Immune Defic Syndr. 2003;32(3):345–346. [PubMed]
14. Skeete K, Hess EP, Clark T, et al. Epidemiology of Suspected Wrist Joint Infection Versus Inflammation. J Hand Surg Am. 2011;36(3):469–474. [PubMed]
15. McCutchan H, Fisher R. Synovial Leukocytosis in Infectious Arthritis. Clin Orthop Relat Res.1990;257:226–230. [PubMed]
16. McGillicuddy D, Shah KH, Friedberg RP, et al. How sensitive is the synovial fluid white blood cell count in diagnosing septic arthritis? Am J Emerg Med. 2007;25(7):749–752. [PubMed]
17. Li S, Henderson J, Dickman E, et al. Laboratory Tests in Adults with Monoarticular Arthritis: Can They Rule Out a Septic Joint? Acad Emerg Med. 2004;11(3):276–280. [PubMed]
18. Kidd B, Hart H, Grigor R. Clinical features of meningococcal arthritis: a report of four cases. Ann Rheum Dis. 1985;44(11):790–792. [PMC free article] [PubMed]
19. Carpenter CR, Schuur JD, Everett WW, et al. Evidence-based diagnostics: adult septic arthritis.Acad Emerg Med. 2011;18(8):781–796. [PMC free article] [PubMed]