| Author | Affiliation |
|---|---|
| Marina Gore, MD | University of Maryland School of Medicine, Department of Emergency Medicine, Baltimore, Maryland |
| Michael E Winters, MD | University of Maryland School of Medicine, Department of Emergency Medicine, Baltimore, Maryland |
ABSTRACT
Erythema gyratum repens (EGR) is a rare and characteristic, paraneoplastic rash associated with a variety of malignancies, most notably lung, esophageal, and breast cancers. This case report details the appearance, epidemiology, diagnosis, and treatment of EGR. Prompt identification of EGR is essential, as the rash often precedes the diagnosis of malignancy by several months. Urgent patient referral to evaluate for malignancy is crucial, as this may lead to decreased morbidity and mortality.
INTRODUCTION
Emergency physicians evaluate patients with rashes almost daily. Many rashes are nonspecific and resolve with minimal treatment, but some skin eruptions are pathognomonic for serious systemic diseases. Erythema gyratum repens (EGR) is a rare, and characteristic, rash strongly associated with malignancy. In this case report, we describe the condition of a 61-year-old woman who presented with a generalized pruritic eruption that was shown to be EGR. We discuss the appearance of EGR, its epidemiology, and its diagnostic and treatment implications.
CASE REPORT
A 61-year-old woman with a history of hypertension and tobacco use presented to our emergency department (ED) with a 6-month history of a generalized rash. She said the rash started as a “bump” on her upper back and gradually spread to involve her torso and extremities. The rash was associated with extreme pruritus, erythema, and scaling (Figures 1 and and 2). Additional symptoms included a 22-lb weight loss, progressive fatigue, anorexia, and intermittent vomiting. The patient recently developed fevers, which prompted her primary care physician to refer her to the ED. During the preceding 2 months, she had taken separate courses of corticosteroids and antifungal medications without improvement in the rash. The only medication she was taking at the time of ED presentation was irbesartan. She had no known allergies, and her family history was negative for connective tissue diseases and malignancy.
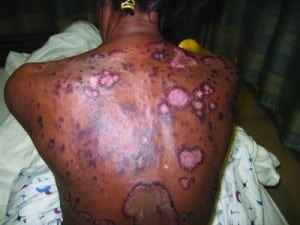
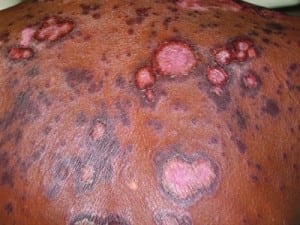
Her vital signs at the time of ED presentation were a temperature of 36°C, pulse of 79 beats/minute, respiratory rate of 16 breaths/minute, pulse oximetry reading of 100% on room air, and blood pressure of 154/89 mmHg. She was not in any distress but appeared uncomfortable because of the rash. Her physical examination was notable for large, well-demarcated, erythematous, plaquelike lesions with raised borders. The lesions demonstrated desquamation and were arranged in concentric circles that covered her thorax, back, abdomen, and extremities. The lesions spared her palms and soles. No bullae, vesicles, or target lesions were observed. The remainder of her examination was remarkable for axillary, inguinal, and anterior and posterior cervical lymphadenopathy.
A complete blood chemistry revealed a white blood cell count of 2.2 K/mcL with 4.5% eosinophils, a hematocrit of 34%, and a platelet count of 120 K/mcL. A comprehensive metabolic panel was significant only for a potassium concentration of 2.7 mEq/L. A rapid plasma reagin test was nonreactive and an anti-nuclear antibody test result was negative. A chest film showed hyperexpansion consistent with chronic obstructive pulmonary disease but otherwise yielded negative results. Computed tomography scans of the head, neck, chest, abdomen, and pelvis demonstrated multiple prominent lymph nodes in the anterior and posterior cervical triangle, bilateral axillary lymphadenopathy, a 9-mm right middle lobe lung nodule, a partially calcified heterogeneous pelvic mass, a tubular soft-tissue density suggestive of right hemipelvic peritoneal mass, and areas of enhanced bone lucencies suggestive of diffuse metastasis. These abnormalities heightened concern for widespread malignancy such as diffuse metastatic carcinoma with an uncertain primary site or, less likely, melanoma or lymphoma.
A dermatology consultation was obtained and a 4-mm punch biopsy specimen was taken from the patient’s left leg. Histopathologic findings demonstrated thick parakeratotic scale and scattered apoptotic cells, consistent with a diagnosis of EGR.
From the ED, the patient was given prescriptions for symptomatic management of her symptoms: hydroxyzine for itching, ibuprofen and oxycodone for pain, and triamcinolone 0.1% cream for the rash. Subsequent outpatient evaluations and procedures led to the diagnosis of adenocarcinoma of gastrointestinal origin. Despite aggressive chemotherapy, the patient died 9 months after her initial ED presentation.
DISCUSSION
Initially described by Gammel1 in 1952 in a patient with metastatic breast cancer, EGR is a paraneoplastic rash associated with a variety of malignancies. The most common are bronchial carcinoma, esophageal cancer, and breast cancer.2–4 In fact, EGR is considered one of the most distinctive cutaneous manifestations of solid tumors.2,4,5 Less commonly, EGR is seen in patients with genitourinary, gastrointestinal, and hematologic malignancies.3,4,6 Rarely, EGR is seen in patients with nonneoplastic conditions such as tuberculosis, hypereosinophilic syndrome, bullous pemphigoid, pemphigus vulgaris, systemic lupus erythematosus, and ulcerative colitis.4,6–10Erythema gyratum repens most often occurs in Caucasians, with a male to female ratio of 2:1. The average age of onset is in the seventh decade of life.2–4 When associated with malignancy, EGR often precedes the diagnosis of cancer by several months.2–5
On examination, EGR has a characteristic appearance consisting of wavy erythematous concentric bands that can be figurate, gyrate, or annular. These bands are arranged in parallel rings and lined by a fine trailing edge of scale, a pattern often described as “wood grained.”2–5,11,12 Erythema gyratum repens can expand as fast as 1 cm a day. It typically involves large areas of the body but tends to spare the face, hands, and feet.2–4 On occasion, bullae form within the areas of erythema.2 The pruritus associated with EGR, an almost universal symptom, can be extreme and debilitating.2–5
Despite the characteristic skin appearance, EGR has nonspecific histopathologic features. Biopsy specimens display acanthosis, mild hyperkeratosis, focal parakeratosis, and spongiosis confined to the epidermis and superficial dermis.2–5 Mononuclear, lymphocytic, and histiocytic perivascular infiltrate in the superficial plexus can also be seen.2,4,5 Basement membrane deposits of IgG, C3, or C4 have been noted under direct immunofluorescence in some cases.4,5
Although the exact cause of EGR is unknown, various immunologic mechanisms have been implicated in its pathogenesis.4,5 One theory is that the tumor may induce antibodies that cross-react with the basement membrane of skin.2,5 Alternatively, some researchers propose that the tumor may produce polypeptides that bind skin antigens and render them immunogenic.13 Still others postulate that deposition of tumor antigen-antibody complexes onto the basement membrane accounts for this reactive dermatitis. The observed immunofluorescence patterns of IgG, C3, and C4 at the basement membrane corroborate a possible immunologic mechanism.2
Although eosinophilia is observed in approximately 60% of cases, the diagnosis of EGR is clinical. Given its distinctive appearance, the differential diagnosis for EGR is usually narrow and includes gyrate erythematous eruptions, such as necrolytic migratory erythema (NME), erythema annulare centrifugum, (EAC) and erythema migrans.2–4 Necrolytic migratory erythema is usually seen in association with pancreatic glucagonomas, favors the perioral and perianal areas, and consists of enlarging erythematous painful annular plaques that form blisters centrally then erode, forming necrotic areas. Lesions of NME typically heal with hyperpigmentation, a characteristic not seen in EGR.3 Erythema annulare centrifugum resembles EGR in that it has pruritic concentric lesions with central clearing and a trailing edge of scale. However, EAC usually involves smaller areas of the trunk and extremities, and it migrates at a much slower rate than the lesions of EGR.2,4 Erythema migrans, the lesion of Lyme disease, begins as a papule at the site of the tick bite and expands peripherally, eventually developing central clearing. Unlike EGR, erythema migrans has no scaling and is a more localized skin reaction.4
Various dermatologic and immunosuppressive therapies have been used to treat EGR. Systemic steroids are frequently ineffective. Topical steroids, vitamin A, and azathioprine have also failed to relieve skin manifestations.2,4 Improvement or resolution of EGR, and its associated intense pruritus, depends on recognition and treatment of the underlying malignancy.2–5,11 In patients with widely metastatic disease, the response of EGR to chemotherapy is variable.2,4 In such cases, patients may not experience resolution of the rash until just before the time of death, a time of significant immunosuppression.2,4,14
CONCLUSION
Many patients present to the ED for evaluation of rashes. Although most rashes are benign and improve with topical therapy alone, several are indicative of more serious pathology. Erythema gyratum repens is not considered a dermatologic emergency in the sense that immediate therapy is required, but it remains a “can’t miss” dermatologic diagnosis. Erythema gyratum repens is a paraneoplastic rash that often precedes the diagnosis of malignancy by several months. With prompt ED recognition, patients can be referred for urgent diagnostic evaluation.
Footnotes
Supervising Section Editor: Rick A. McPheeters, DO
Submission history: Submitted September 7, 2010; Revision received October 28, 2010; Accepted November 1, 2010
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2010.11.2090
Address for Correspondence: Michael E. Winters, MD
University of Maryland School of Medicine, Department of Emergency Medicine, 110 S Paca St, 6th Floor, Ste 200, Baltimore, MD 21201
E-mail: mwint001@umaryland.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Gammel JA. Erythema gyratum repens. Arch Dermatol Syph. 1952;;66:494–505.
2. Stone SP, Buescher LS. Life-threatening paraneoplastic cutaneous syndromes. Clin Dermatol.2005;23:301–306. [PubMed]
3. Chung VQ, Moschella SL, Zembowicz A, et al. Clinical and pathologic findings of paraneoplastic dermatoses. J Am Acad Dermatol. 2006;;54:745–762. [PubMed]
4. Eubanks LE, McBurney E, Reed R. Erythema gyratum repens. Am J Med Sci. 2001;;321:302–305.[PubMed]
5. Kleyn CE, Lai-Cheong JE, Bell HK. Cutaneous manifestations of internal malignancy. Am J Clin Dermatol. 2006;;7:71–84. [PubMed]
6. Kiyohara T, Kumakiri M, Kobayashi H, et al. Erythema gyratum repens accompanied by essential thrombocythemia, followed by a blastic crisis. Acta Derm Venereol. 2003;;83:133–134. [PubMed]
7. Barber PV, Doyle L, Vickers DM, et al. Erythema gyratum repens with pulmonary tuberculosis. Br J Dermatol. 1978;;98:465–468. [PubMed]
8. Morita A, Sakakibara N, Tsuji T. Erythema gyratum repens associated with hypereosinophilic syndrome. J Dermatol. 1994;;21:612–614. [PubMed]
9. Durani BK, Andrassy K, Hartschuh W. Neutrophilic dermatosis with an erythema gyratum repens-like pattern in systemic lupus erythematosus. Acta Derm Venerol. 2005;;85:455–456. [PubMed]
10. España A, Sitaru C, Pretel M, et al. Erythema gyratum repens-like eruption in a patient with epidermolysis bullosa acquisita associated with ulcerative colitis. Br J Dermatol. 2007;;127:773–775.
11. Boyd AS, Neldner KH, Menter A. Erythema gyratum repens: a paraneoplastic eruption. J Am Acad Dermatol. 1992;;26:757–762. [PubMed]
12. Kawakami T, Saito R. Erythema gyratum repens unassociated with underlying malignancy. J Dermatol. 1995;;22:587–589. [PubMed]
13. Holt PJ, Davies MG. Erythema gyratum repens—an immunologically mediated dermatosis? Br J Dermatol. 1997;;96:343–347. [PubMed]
14. Skolnick M, Mainman ER. Erythema gyratum repens with metastatic adenocarcinoma. Arch Dermatol. 1975;;111:227–229. [PubMed]


