| Author | Affiliation |
|---|---|
| Malford T Pillow, MD | Baylor College of Medicine, Section of Emergency Medicine, Houston, Texas |
| Ngoc Anh Nguyen, MD | Baylor College of Medicine, Section of Emergency Medicine, Houston, Texas |
| Dick Kuo, MD | Baylor College of Medicine, Section of Emergency Medicine, Houston, Texas |
ABSTRACT
We present the case of a 32-year-old woman who presented to the emergency department with a witnessed cardiac arrest. She was otherwise healthy with no cardiac risk factors and had undergone an uneventful repeated cesarean section 3 days priorly. The patient underwent defibrillation, out of ventricular fibrillation to a perfusing sinus rhythm, and was taken to the catheterization laboratory where coronary angiography findings showed spontaneous dissection of the left anterior descending artery. The patient received a total of 6 stents during her hospital stay and was eventually discharged in good condition. Spontaneous coronary artery dissection is a rare entity with a predilection for pregnant or postpartum women. Early diagnosis and treatment are key for survival, and when identified early, mortality rate is reduced.
INTRODUCTION
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute myocardial infarction (MI) in the general population, especially in younger patients without classic coronary risk factors.1However, there have been many documented cases of SCAD causing acute MI in pregnant or postpartum women, a condition referred to as “pregnancy-associated coronary artery dissection” (PACAD).2 Mortality rate for PACAD was reported to be as high as 66%, in a review by Engelman et al3 in 1993, but a more recent analysis by Koul et al4 in 2001 has demonstrated a mortality rate of 38%, likely due to earlier diagnosis and intervention. We present the case of a 32-year-old woman who presented in cardiac arrest and was ultimately diagnosed with PACAD, as well as a review of the literature. To our knowledge, this is the first case of PACAD presenting as cardiac arrest reported in the literature.
CASE PRESENTATION
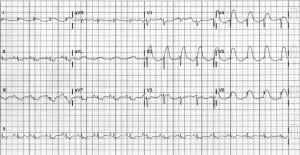
A 32-year-old Hispanic female with no past medical problems presented to the Ben Taub Emergency Department with shortness of breath and severe chest pain. She was G2P2A0 and 3 days earlier, she had undergone an uneventful repeated cesarean section. While undergoing triage, the patient lost consciousness and became unresponsive. She was found to be pulseless and apneic and her initial rhythm was ventricular fibrillation. Advanced cardiac life support protocols were started and the patient underwent defibrillation twice before conversion to a sinus rhythm. The postresuscitation 12-lead electrocardiogram (ECG) showed ST-segment elevations in the anterior leads with reciprocal ST depressions (see Figure). At that point, she was transported emergently to the cardiac catheterization laboratory and was found to have dissection of the left anterior descending artery (LAD), and 3 stents were placed. During catheterization, she required temporary transvenous pacing and an intra-aortic balloon pump (IABP). After stabilization, she was transferred to the cardiac intensive care unit (CCU). Her systolic blood pressure stayed above 100 mm Hg with inotropic support. One day later, the patient reconverted to ventricular fibrillation and required another defibrillation. She was returned to the catheterization laboratory a second time and was noted to have proximal dissection of the LAD beyond the initial stents placed and received 3 more stents for a total of 6 stents. She was returned to the CCU, again requiring inotropes, temporary pacing, and IABP to manage the cardiogenic shock. Over the next several days, the patient slowly improved and was weaned off inotropes. The IABP was removed and she underwent extubation.
After the initial acute phase of hospitalization, the patient continued to remain hemodynamically stable. The chest radiograph results showed gradual improvement of her condition with remission from respiratory failure. Her hemoglobin levels and hematocrit remained stable during the latter part of her admission. She was able to ambulate and began working with physical therapy for rehabilitation exercises. She was eventually discharged with vital signs within normal limits. Her predischarge echocardiogram showed a left ventricular ejection fraction of 30% to 34%, which was unchanged from an echocardiogram done earlier during her hospitalization. The echocardiogram showed hypokinesis of the anterior wall of left ventricle, but no left ventricular wall thrombus. She was discharged in good condition and given Aspirin, Plavix, Lisinopril, Coreg, and Aldactone.
One month later, the patient again presented to the emergency department complaining of intermittent chest pressure for 2 days, which was not associated with shortness of breath or sweating. Her vital signs were normal and cardiovascular, pulmonary, abdominal, musculoskeletal, and genitourinary examination results were normal. Cardiac enzyme levels were within normal limits, and ECG showed T-wave inversions V3-V6, Q waves in V1-V4, but no acute changes from the discharge ECG. Three sets of cardiac enzyme results remained negative. All ECG changes were consistent with the post MI ECGs. Exercise stress test was ordered, and the patient tolerated 9 minutes of exercise, with no ischemia. She was further evaluated by the cardiology service and subsequently discharged and given Aspirin, Lisinopril, Coreg, and Plavix.
DISCUSSION
Acute myocardial infarction (AMI) is a rare occurrence in women of childbearing age, given the relatively young age of the population.5 However, pregnancy increases the risk of AMI threefold to fourfold. According to a study of the population in 2000–2002 by James et al,5 AMI occurs in about 6.2 cases per 100,000 deliveries.
Of the cases of AMI in pregnant and postpartum women, SCAD accounted for 27% of cases compared to 1% or fewer in the general population.6,7 Although rare, these incidences are notable because the mortality rate is usually high, ranging from 38% to 66%.3,4 Presumably, the lower mortality rate found by Koul and colleagues4 is an effect of earlier interventions and improvements in treatment of MI in the subsequent years.
The cause of increased rates of AMI and SCAD in pregnant and postpartum women has not been elucidated. Some theories attribute these negative outcomes to hormonal and hemodynamic changes in pregnancy, such as increase in cardiac output, decreased collagen production, disruption of the vaso vasorum, and alterations in the coagulation-fibrinolysis system.6 These increased stresses on the arterial system, coupled with increased coagulability and decreased collagen production, are thought to predispose to SCAD, despite the patient’s not having previous classic coronary risk factors. In postpartum patients, postpartum degeneration of the ground substance of the connective tissue in the intima and media is thought to contribute to the artery’s inability to handle the shear stress of increased cardiac output.8 The mechanism of decreased collagen production is thought to be similar to defects seen in connective tissue diseases such as Marfan and Ehler-Danlos. This would also explain the increased incidence of spontaneous dissection in these populations.6
Management of the pregnant or postpartum patient depends on the hemodynamic status of the patient, viability of the fetus, and the extent of myocardial damage. Three notable cases in the literature describe pregnant or postpartum patients who had full recovery after conservative treatment.9–11 Of note, thrombolytic therapy has been used in the past but has lost favor owing to potential complications such as postpartum hemorrhage or fetal intracranial hemorrhage.12,13 In addition, patients have been successfully treated with drug-eluting stents, as in our patient’s case.14,15 In more severe cases in which the patient undergoes cardiogenic shock with an extensive amount of injured myocardium, coronary artery bypass has been performed with good results.16,17Interestingly, the review done by Koul and colleagues4 demonstrated that once the diagnosis is made, the mortality rate drops to 0% regardless of the therapy chosen. A more recent literature review conducted by Shamloo and colleagues17 demonstrates a more favorable outcome when aggressive treatments (cardiac catheterization or coronary artery bypass graft) are performed versus conservative medical management.
The first case of SCAD was documented in 1931 by Pretty18 on an autopsy performed on a 42-year-old female who presented with chest pain. We conducted a literature review of all cases of PACAD from 1931 to 2010. Our literature search found 104 cases of documented spontaneous coronary artery dissection in the peripartum period, with 47 patients (45% of total) presenting in cardiac arrest and 18 cases (17% of total) documenting a progression of the dissection or recurrent dissection in a different location. Of the patients presenting in cardiac arrest, 42 of 47 presented in sudden cardiac death, and 5 of 47 presented in ventricular fibrillation.4,20–22 Of the 5 patients who presented in ventricular fibrillation, only 3 survived.4 Although our patient presented in ventricular fibrillation and cardiac arrest, with immediate defibrillation and timely cardiac catheterization, she survived.
Of the documented cases of progression of disease, 13 of 18 demonstrated recurrent dissections in a different location from the original dissection.20,21,23–25 The recurrent dissections occurred during the same hospital stay and up to 2 months apart from each other.24 Five of the 18 cases described a progression of the same dissection. In all 5 cases, the patient was treated medically at first and then had to be treated by a more invasive method, such as stenting or coronary artery bypass graft.6 Our case is notable because the patient presented with a witnessed cardiac arrest. Her initial postresuscitation ECG showed ST-segment elevations consistent with an anterior MI, making PACAD the most likely diagnosis. She received 3 stents, and her clinical course was complicated by cardiogenic shock necessitating pressor support, transvenous pacing, and IABP. She developed recurrent dysrhythmia and was found to have proximal progression of the dissection, necessitating 3 more stents for a total of 6 stents in the LAD.
Pregnancy-associated coronary artery dissection should serve as a reminder to cardiologists, emergency medicine physicians, and obstetricians to consider early coronary artery angiography for diagnosis in women with complaints of chest pain, even with no risk factors. Percutaneous coronary angiography (PTCA) and coronary artery bypass graft have both been shown to be effective, although indications for one over the other are unclear. However, owing to both the diagnostic and potentially therapeutic benefits of PTCA, it is becoming the first line of treatment in suspected PACAD. Conservative treatment has shown to be effective in hemodynamically stable patients with lesions not conducive to stenting.
Footnotes
Supervising Section Editor: Rick A. McPheeters, DO
Submission history: Submitted March 24, 2011; Revision received April 12, 2011; Accepted April 15, 2011
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.4.2263
Address for Correspondence: Malford T. Pillow, MD
Baylor College of Medicine, Section of Emergency Medicine, 1504 Taub Loop, Mail Stop 285, Houston, TX 77004
E-mail: tysonpillow@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Thompson EA, Ferraris S, Gress T, et al. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of the reported cases. J Invasive Cardiol.2005;;17:59–61. [PubMed]
2. Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. Ann Int Med.1996;;22:19–37.
3. Engelman DT, Thayer J, Derossi J, et al. Pregnancy related coronary artery dissection: a case report and collective review. Conn Med. 1993;;57:135–139. [PubMed]
4. Koul AK, Hollander G, Moskovits N, et al. Coronary artery dissection during pregnancy and the postpartum period: two case reports and review of literature. Catheter Cardiovasc Interv.2001;;52:88–94. [PubMed]
5. James AH, Jamison MG, Biswas MS, et al. Acute myocardial infarction in pregnancy: a Untied States population-based study. Circulation. 2006;;113:1564–1571. [PubMed]
6. Appleby CE, Barolet A, Ing D, et al. Contemporary management of pregnancy-related coronary artery dissection: a single-centre experience and literature review. Exp Clin Cardiol. 2009;;14:e8–e18. [PMC free article] [PubMed]
7. Hering D, Piper C, Hohmann C, et al. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Zeitschrift fur Kardiologie. 1998;87:961–970. [PubMed]
8. Mather PJ, Hansen CL, Goldman B, et al. Postpartum multivessel coronary dissection. J Heart Lung Transplant. 1994;;13:533–537. [PubMed]
9. Satoda M, Takagi K, Uesugi M, et al. Acute myocardial infarction caused by spontaneous postpartum coronary artery dissection. Nat Clin Prac Cardiovasc Med. 2007;;4:688–692.
10. Van den Branden BJ, Bruggeling WA, Corbeij HM, et al. Spontaneous coronary artery dissection in the postpartum period. Neth Heart J. 2008;;16:412–414. [PMC free article] [PubMed]
11. Vogiatzis I, Hadjimitiades S, Sachpekidis V, et al. Spontaneous coronary artery dissection and acute myocardial infarction during pregnancy. Hellenic J Cardiol. 2010;;51:74–80. [PubMed]
12. Madu EC, Kosinski DJ, Wilson WR, et al. Two-vessel coronary dissection in the peripartum period: case report and literature review. Angiology. 1994;;45:809–816. [PubMed]
13. Sebastian C, Scherlag M, Kugelmass A, et al. Primary stent implantation for acute myocardial infarction during pregnancy: use of abciximab, ticlopidine, and aspirin. Cathet Cardiovasc Diagn.1998;;45:275–279. [PubMed]
14. Azarelli S, Fiscella D, Amico F, et al. Multivessel spontaneous coronary artery dissection in a postpartum woman treated with multiple drug-eluting stents. J Cardiovasc Med. 2009;;10:340–343.
15. Mohamed HA, Eshwesh A, Habib N. Spontaneous coronary artery dissection—a case report and review of the literature. Angiology. 2002;;53:205–211. [PubMed]
16. Goland S, Schwarz ER, Siegel RJ, et al. Pregnancy-associated spontaneous coronary artery dissection. Am J Obstet Gynecol. 2007;;197:e11–e13. [PubMed]
17. Shamloo BK, Chintala RS, Nasur A, et al. Spontaneous coronary artery dissection: aggressive vs conservative therapy. J Invasive Cardiol. 2010;;22:222–228. [PubMed]
18. Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. Br Med J. 1931;;1:667.
19. Kelly P, Barrett C. Coronary artery disease in puerperium. Eur J Med. 2005;;16:469–472.
20. Koller PT, Cliffe CM, Ridley DJ. Immunosuppressive therapy for peripartum-type spontaneous coronary artery dissection: case report and review. Clin Cardiol. 1998;;21:40–46. [PubMed]
21. Robinowitz M, Virmani R, McAllister HA. Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am J Med. 1982;;72:923–928. [PubMed]
22. Bonnet J, Aumailley M, Thomas D, et al. Spontaneous coronary artery dissection: case report and evidence for a defect in collagen metabolism. Eur Heart J. 1986;;7:904–909. [PubMed]
23. Togni M, Amann FW, Follath F. Spontaneous multivessel coronary artery dissection in a pregnant woman treated successfully with stent implantation. Am J Med. 1999;;107:407–408.[PubMed]
24. Marcoff L, Popescu A, Price L, et al. Spontaneous coronary artery dissection in a postpartum woman presenting with chest pain. Am J Emerg Med. 2010;;28:980–981.
25. Schroder C, Stoler RC, Branning GB, et al. Postpartum spontaneous coronary artery dissection confirmed by coronary CT angiography. Proc (Bayl Univ Med Cent) 2006;;19:338–341.[PMC free article] [PubMed]


