| Author | Affiliation |
|---|---|
| Matthew A. Wheatley, MD | Emory University, Department of Emergency Medicine, Atlanta, GA |
| Bijal B. Shah, MD | Emory University, Department of Emergency Medicine, Atlanta, GA |
| Brent W. Morgan, MD | Emory University, Department of Emergency Medicine, Atlanta, GA |
| Debra Houry, MD, MPH | Emory University, Department of Emergency Medicine, Atlanta, GA |
| Ziad N. Kazzi, MD | Emory University, Department of Emergency Medicine, Atlanta, GA |
ABSTRACT
Introduction:
Poisoning is an increasingly important cause of injury in the United States. In 2009 poison centers received 2,479,355 exposure reports, underscoring the role of poison centers in intentional and unintentional injury prevention. Antiretroviral (ARV) agents are commonly prescribed drugs known to cause toxicity, yet the frequency of these incidents is unknown. The objectives of this study were to quantify the number of reported cases of toxicity secondary to ARV agents at a regional poison center, and to describe the circumstances and clinical manifestations of these poisonings.
Methods:
We conducted a retrospective review of poison center records between December 1, 2001, and January 7, 2010.
Results:
One hundred sixty-two exposures to ARV agents were reported to the poison center, of which 30% were intentional and 70% were unintentional. Three patients developed major toxicity and no deaths occurred. The remaining patients developed moderate and minor effects as defined by poison center guidelines.
Conclusion:
ARV drug toxicity appears to be infrequently reported to the poison center. Fatal and major toxicities are uncommon, and intentional overdoses are associated with a more serious toxicity. Educational efforts should encourage clinicians to report toxicities related to the use of ARV agents to poison centers in order to better study this problem.
INTRODUCTION
Poisoning, an important cause of morbidity and mortality in the United States (U.S.), can be intentional or unintentional. Poison centers receive reports from the public, as well as healthcare professionals, for poisoning exposures in the 50 states and U.S. territories.1 The data is compiled and monitored in a near-real time setting through the National Poison Data System.2
Antiretroviral agents (ARVs) are commonly prescribed drugs with well-characterized adverse effects. They are effective in improving survival rates for patients with human immunodeficiency virus and acquired immunodeficiency syndrome.3 However, chronic adverse effects, such as pancreatitis, hypersensitivity, peripheral neuropathy and nephrolithiasis, can limit clinical effectiveness and in some cases may be fatal. Lactic acidosis is the most serious adverse effect. It can be seen in the setting of acute overdose, as well as with chronic therapeutic use, and has a mortality of 33–57%.4
The true incidence of intentional or unintentional injury due to ARVs is unknown. A total of 5,563 exposures to ARVs were reported to the American Association of Poison Control Centers between 2001 and 2008, of which 29% were intentional. Five deaths were reported and 4% of patients had major clinical outcomes.2
This study will quantify the number of intentional and unintentional injuries due to toxic exposures to ARV medications reported to the Georgia Poison Center (GPC).
METHODS
The GPC is located in Atlanta and serves the state of Georgia. It receives more than 80,000 exposure calls per year and has one of the largest call volumes when compared to the rest of the nation’s poison centers. Specialists in Poison Information Systems (SPIS) answer all incoming calls from both persons in the community and medical professionals. Their tasks are to record pertinent information about the poisoning and make recommendations regarding management. The patient’s name, age and gender are recorded, as well the names of all substances involved, amount, route, circumstances, time of exposure and symptoms. These data are entered into the poison center database using a combination of specific data fields and free text space.
Based on information from the initial phone call, the SPIS classify clinical outcome due to the exposure as a no effect, minor, moderate, major outcome or death. Case outcomes are classified as minor when the effect involves only one organ system, is short in duration and has no permanent effects. Effects that are more prolonged (as judged by the SPIS) or involve more than one organ system are termed moderate outcomes. Major outcomes involve permanent disability or life-threatening exposures. Exposures that do not meet these criteria are deemed to have no potential to be toxic, or not related to the reported clinical effect, and are not followed to an outcome.
The poison center attempts to follow all exposures involving minor, moderate or major effects to outcome via phone calls to the patient or treating facility. Any additional information obtained from these phone calls is entered into the poison center database in the same fashion as the initial call. Occasionally, the poison center is unable to obtain further information about the case.
Two study investigators accessed the poison center database in July 2009, and January 2010. They queried for entries involving any ARV agent from December 1, 2001, through the date accessed, most recently, January 7, 2010. Information obtained for each case included patient age, gender, substances ingested, effects, therapies administered, management site and medical outcome (when available). We performed descriptive statistics on demographics, type of medication and category of outcome after overdose. The local institutional review committee approved this study.
RESULTS
One hundred sixty-two exposures were documented between December 1, 2001 and January 7, 2010, with 15 patients lost to follow up. The average patient age was 27-years-old (standard deviation [SD] 19 years) and 100 cases (62%) were male. Zidovudine was the most common single drug reported as causing toxicity. It was involved in 46 patients as a single or combination drug.
Of the 162 exposures, 49 (30%) were judged intentional and 113 (70%) unintentional or unknown. We classified intentional exposures as suspected suicide (96%) or intentional misuse (4%). Unintentional exposures were classified as therapeutic error (58%), general unintentional (33%), adverse drug reactions (8%) or unknown (1%). General unintentional exposures are those not otherwise categorized as adverse drug reaction, therapeutic error, or unknown.
Of the 162 patients, 51 (31%) were categorized as potentially toxic or related to the clinical effect and were followed to clinical outcome. Thirty-four (67%) of these patients had intentional exposures. Of the 51 patients that were followed to a clinical outcome, 6% had a major, 27% had a moderate, 23% had a minor, and 51% had no effect. There were no patient deaths. Table 1 summarizes the above findings.

The three patients who had a major effect were admitted to a critical care unit. Two of these cases were reported as intentional ingestions and the third was an unknown exposure. One exposure involved Trizivir® which contains abacavir, lamivudine and zidovudine. This patient developed coma. A second exposure was to lamivudine (Epivir®) 300mg tablets, efavirenz (Sustiva®) 200mg capsules and abacavir (Ziagen®) 300 mg tablets. This patient developed lactic acidosis, renal failure and coma. The final case with major toxicity involved exposure to stavudine alone. This patient developed renal failure and coma. The number of pills ingested and non-ARV co-ingestants were not reported to the GPC for these cases.
Among patients categorized with moderate effects, six reported exposure to multiple ARVs. Six patients were admitted to critical care units, three to psychiatric facilities, and the others required minimal medical treatment. The most common effects reported in this group were hypertension (six patients), tachycardia (four patients), agitation (three patients), hypotension (three patients) and vomiting (three patients).
Thirteen patients were reported to have minor effects. One patient had a three-drug exposure and four patients had a two-drug exposure. The most common effects reported in this group were drowsiness or lethargy (four patients), abdominal pain (three patients) and vomiting (three patients).
Excluding the 15 patients that were lost to follow up, 54% of the intentional exposures had a major, moderate, or minor effect. This is in comparison to 8% of unintentional exposures.
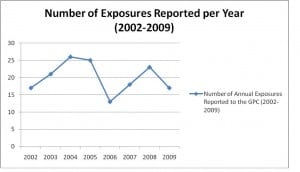
DISCUSSION
Overall, exposures to ARVs comprise a small percent of the annual exposures reported to the GPC. There is no discernable trend in the number of exposures reported per year (Figure 1). There were a small number of cases of toxicity from ARV medications reported as well. Furthermore, only a small percentage of the total reported cases (2% or three patients) led to major toxicity. Due to our low numbers of critically ill patients, we are unable to predict which patients are at risk for severe morbidity or mortality. Based on our data, the majority of exposures to ARVs, whether intentional or unintentional, led to self-limited or no clinical effects.
As reported above, 54% of patients with intentional exposures had clinical effects, compared with 8% of patients with unintentional exposures. This results from larger doses of medications ingested by patients with intentional exposures compared to those who have adverse reactions due to therapeutic usage. Unfortunately, information on ingested dose is not consistently available.
In 2008, 60 U.S. Poison Centers reported receiving 4,333,012 exposure calls from the public or health care providers.2 Although ARVs represent a small proportion of the reported exposures, the potential for serious injury due to these agents remains and is a public health concern. Clinicians should be encouraged to report all injuries due to pharmaceuticals to poison centers. This will help organizations, such as the AAPC, to construct less subjective data-collection tools. This could lead to more sensitive monitoring of trends of side effects of specific therapeutic agents or the use of certain pharmaceuticals, such as ARVs, for intentional injury. In this current era of preventive medicine and drug safety, a comprehensive, and rigorous, real-time surveillance system will prove invaluable to healthcare and public health. Policy makers and pharmaceutical companies can then use this information to minimize risks posed to patients by these drugs.
LIMITATIONS
There are several limitations to this study. First, it was a retrospective analysis of relatively few cases. Cases of ARV toxicity may have been misclassified as an unknown ingestion. Poison center data have been shown to be prone to incompleteness and inaccuracy. Second, some of the compounds involved in exposures reported to the GPC have been introduced within the last 10 years, meaning less data exist on toxicities due to intentional or unintentional overdose for these compounds in the clinical setting. Furthermore, with fewer than 10 exposures reported for many of these compounds, drawing a link between exposure and outcomes is not possible. Moreover, the reported dose ingested is often unknown or uncertain. Lastly, we had 15 patients lost to follow-up.
CONCLUSION
Major toxicity and death due to ARV medication are rare occurrences, as currently reported to the GPC. Patients with intentional exposures were more likely to have clinical effects than patients with unintentional exposures. Clinicians should be encouraged to report suspected intentional or unintentional injury due to pharmaceuticals to their local poison center. In the future, policy makers can mandate reporting injuries by healthcare professionals from commonly used and or potentially toxic medications and greatly enhance the accuracy of existing surveillance systems.
Footnotes
Supervising Section Editor: Abigail Hankin, MD, MPH
Submission history: Submitted January 17, 2011; Revision received February 28, 2011; Accepted March 3, 2011
Reprints available through open access at http://scholarship.org/uc/uciem_westjem.
Address for Correspondence: Matthew A. Wheatley MD
49 Jesse Hill Jr. Drive SE, Atlanta, GA 30303
E-mail: mwheatl@emory.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Bronstein AC, Spyker DA, Cantilena LR, et al. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clinical Toxicology. 2010;48:979–1178. [PubMed]
2. The American Association of Poison Control Centers Web site. Available at:http://www.aapcc.org.dnn/Portals/0/2008annualreport.pdf. Accessed January 31, 2010.
3. Palella FJ, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138(8):620–6. [PubMed]
4. Falcó V, Rodriguez D, Ribera E, et al. Severe nucleoside-associated lactic acidosis in human immunodeficiency virus-infected patients: Report of 12 cases and review of the literature. Clin Infect Dis. 2002;34(6):838–46. [PubMed]


