| Author | Affiliation |
|---|---|
| J. Shaun Smith, DO | Ohio State University Medical Center, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Columbus, OH |
| Jennnifer W. McCallister, MD | Ohio State University Medical Center, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Columbus, OH |
A 66-year-old Caucasian male with no past medical history presented to the emergency department (ED) complaining of severe retrosternal pain, which began immediately prior to arrival following an episode of vomiting. The patient had symptoms including nausea, vomiting and generalized abdominal discomfort for three days prior. The patient had been evaluated by a primary care physician earlier the day of presentation and was diagnosed with gastroenteritis and possible early small bowel obstruction per computed tomography (CT) scan of the abdomen and pelvis. The patient was released home with anti-emetics. On presentation to the ED the patient was in moderate distress secondary to pain and hemodynamically stable. Physical exam was remarkable for absent breath sounds over the left hemithorax. Chest radiography revealed a left-sided pneumo-hydrothorax (Figure 1). Subsequent CT of the chest also demonstrated pneumo-hydrothorax, as well as mediastinal air (Figure 2). Laboratory studies showed normal comprehensive metabolic panel and coagulation profile; however, the CK-MB was elevated at 6.70 ng/ml (3.60–5.00), and a leukocytosis of 11.9 TH/ul (4.0–9.0). Electrocardiograph showed normal sinus rhythm with a ventricular rate of 87. A 28 French chest tube was inserted, draining 600 mL of dark blood-tinged fluid. Based on radiographic findings and clinical presentation, esophageal rupture was the primary diagnosis. The patient was transferred to a tertiary center where Boerhaave’s was confirmed with a barium esophagram. Surgical repair was successfully performed.
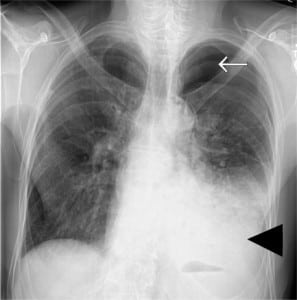
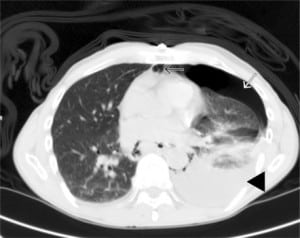
Patients classically present with chest pain, vomiting and subcutaneous emphysema. Pain may radiate to the neck, arm or back and is aggravated by deep breathing or swallowing. A myriad of other findings may be present, including hoarseness, JVD, and cyanosis. The Hamman crunch may be ascultated. A distinctive sound corresponding with each heartbeat is indicative of pneumomediastinum.1 As time progresses, the clinical picture can be clouded by sepsis syndrome. Chest radiography is the preferred initial study and is most often abnormal, showing mediastinal or free peritoneal air. CT scan may reveal periesophageal fluid with possible air bubbles, pneumo-hydrothorax, esophageal wall edema, or widened mediastinum. Water-soluble esophagram is performed to confirm rupture; if negative, one should pursue a barium study treatment based on the location of the perforation. Thoracic esophageal perforations require surgical intervention within the first 24 hours involving mediastinal and chest drainage and esophageal repair. Cervical esophageal perforations can often be managed non-surgically.2 Underlying esophageal disease may also influence the type of therapy, such that the treatment of the underlying process is included in the repair of the perforation. In one study morbidity and mortality of patients with underlying esophageal pathology were similar to patients without prior disease.3 Concomitant therapies include antibiotics, acid suppression, nasogastric suction and supplementation, such as total parenteral nutrition or enternal feeding.
Footnotes
Supervising Section Editor: Sean Henderson, MD
Submission history: Submitted July 21, 2009; Revision Received September 2, 2009; Accepted September 14, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Jennifer W. McCallister, MD, The Ohio State University Medical Center Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, 201 Davis Heart and Lung Research Institute, 473 West 12th Avenue, Columbus, OH 43210
Email: Jennifer.McCallister@osumc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Janjua KJ. Boerhaave’s syndrome. Postgrad Med J. 1997;73(859):265–70. [PMC free article][PubMed]
2. Amir AI, van Dullemen H, Plukker JT. Selective approach in the treatment of esophageal perforations. Scand J Gastroenterol. 2004;39(5):418–22. [PubMed]
3. Atallah FN, Riu BM, Nguyen LB, et al. Boerhaave’s syndrome after postoperative vomiting. Anesth Analg. 2004;98(4):1164–6. [PubMed]


