| Author | Affiliation |
|---|---|
| David E. Slattery, MD | University of Nevada School of Medicine, Department of Emergency Medicine, Las Vegas, NV |
| Charles V. Pollack, MA, MD | niversity of Pennsylvania, Department of Emergency Medicine, Philadelphia, PA |
ABSTRACT
Introduction:
To review available evidence and examine issues surrounding the use of advanced antiplatelet therapy in an effort to provide a practical guide for emergency physicians caring for patients with acute coronary syndromes (ACS).
Methods:
American College of Cardiology/American Heart Association (ACC/AHA) 2007 guidelines for the management of patients with unstable angina (UA) and non-ST-segment elevation myocardial infarction (NSTEMI), AHA/ACC 2007 focused update for the management of patients with STEMI, selected clinical articles identified through the PubMed database (1965-February 2008), and manual searches for relevant articles identified from those retrieved.
Results:
Clinical data, including treatment regimens and patient demographics and outcomes, were extracted and critically analyzed from the selected studies and clinical trials. Pertinent data from relevant patient registries were also evaluated to assess current clinical practice.
Conclusion:
As platelet activation and aggregation are central to ACS pathology, antiplatelet agents are critical to early treatment. A widely accepted first-line treatment is aspirin, which acts to decrease platelet activation via inhibition of thromboxane A2 synthesis. Thienopyridines, which inhibit ADP-induced platelet activation, and glycoprotein (GP) receptor antagonists, which bind to platelet GP IIb/IIIa receptors and hinder their role in platelet aggregation and thrombus formation, provide complementary mechanisms of platelet inhibition and are often employed in combination with aspirin. While the higher levels of platelet inhibition that accompany combination therapy improve protection against ischemic and peri-procedural events, the risk of bleeding is also increased. Thus, the challenge in choosing appropriate therapy in the emergency department lies in balancing the need for potent platelet inhibition with the potential for increased risk of bleeding and future interventions the patient is likely to receive during the index hospitalization.
INTRODUCTION
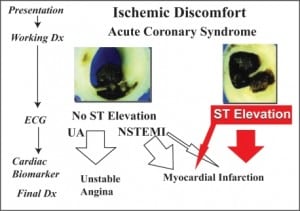
Acute coronary syndrome (ACS) describes a spectrum of atherothrombosis, including unstable angina (UA), non−ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI). As treatment decisions are driven by ACS type and severity, initial risk stratification in the emergency department (ED) is essential. In addition to historical factors and hemodynamic stability, electrocardiographic and cardiac biomarker findings play an important role in differentiating UA/NSTEMI from STEMI (Figure 1). Patients with acute STEMI are candidates for immediate reperfusion therapy with adjunctive antiplatelet and antithrombotic therapy. The optimal strategy (percutaneous coronary intervention [PCI] vs. fibrinolysis) depends on the patient’s clinical condition, timing of presentation, and the availability of interventional resources. In patients with UA/NSTEMI, diagnostic tools such as the 7-point Thrombolysis in Myocardial Infarction (TIMI) risk score can be used for semi-quantitative assessment of the risk of cardiac ischemic complications, where the risk of mortality or adverse cardiovascular events increases with the scale score.1 It is recommended that high- and intermediate-risk UA/NSTEMI patients be managed with an early invasive strategy (i.e., diagnostic angiography followed by revascularization [PCI or coronary artery bypass graft (CABG)]).2,3 The choice of optimal revascularization depends on the patient’s coronary anatomy, left ventricular function, and the presence of co-morbidities such as diabetes. Lower-risk patients can receive medical management, with diagnostic angiography deferred unless deterioration occurs.3
Since platelet activation and aggregation are pivotal to ACS pathology, antiplatelet therapy, including aspirin, thienopyridines and glycoprotein IIb/IIIa receptor inhibitors (GPIs), is central to ACS treatment. Aspirin, which inhibits platelet activation by irreversibly binding to cyclooxygenase-1, is widely accepted as first-line treatment in ACS patients.2 By irreversibly binding the platelet P2Y12 receptor, thienopyridines inhibit adenosine disphosphate-mediated platelet activation. GPIs prevent activated platelets from cross-linking with fibrinogen, and ultimately decrease the trapping of red blood cells that leads to early vessel thrombus formation, obstruction, and/or distal small vessel embolization. “Dual” antiplatelet therapy (aspirin plus GPIs or aspirin plus thienopyridines) is appropriate in some patients, while in others, “triple” therapy including all three agents is suitable.
Emergency physicians (EPs) must choose appropriate antiplatelet therapy based on the underlying risk of ischemic complications and the anticipated course of treatment, i.e. medical vs. interventional management.4 Ideally, evidence-based, predetermined ACS protocols should be in place so that optimal antiplatelet therapy can occur concurrently with maximum protection against bleeding complications. Ongoing collaboration among EPs, cardiologists, hospitalists and cardiovascular surgeons will undoubtedly improve care for ACS patients. Data from CRUSADE, a national health quality improvement initiative, showed significant improvement in adherence to guideline recommendations for ACS management in the acute setting, as participating hospitals developed more thorough cross-disciplinary pathways and protocols.5 It is through such institutional-level collaboration that EPs can be empowered to initiate early, appropriate anti-ischemic therapy rather than being dependent on the individual, often varied, preferences of on-call specialists.
In addition to disease-related ischemia and necrosis, high-risk patients who undergo angiography face the potential added burden of periprocedural ischemia. It is hypothesized that microvascular embolization downstream of the target vessel plays a predominant role in the development of periprocedural infarction risk.6 Hence, it is important to recognize the adjuvant role of pre-catheterization (“upstream”) antiplatelet agents and anticoagulation during coronary intervention to offer protection against both disease-related and periprocedural ischemic insults. Appropriate therapy must balance the need for potent platelet inhibition with the potential for increased bleeding.
This review aims to examine issues and barriers surrounding antiplatelet therapy use and to provide a practical guide for EPs regarding their optimal use in ACS patients. While not a purely systematic review, we sought to identify relevant controlled studies and randomized clinical trials that assessed the efficacy and safety of antiplatelet therapy in treating patients with all ACS manifestations. Other data sources included 1) the 2007 American College of Cardiology/American Heart Association (ACC/AHA) ACS treatment guidelines, 2) relevant clinical data extracted from patient registries, and 3) selected clinical articles identified through the PubMed database (1965-February 2008) using appropriate search terms (e.g. acute coronary syndrome, antiplatelet agents, atherosclerosis, blood platelets, myocardial infarction, thrombosis).
Variability of platelet response
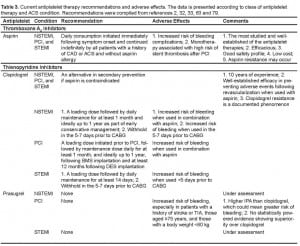
Available secondary prevention therapies do not provide cures. They decrease associated risks. Despite receiving “adequate” antiplatelet therapy, approximately 8–10% of patients experience recurrent cardiovascular ischemic events after ACS.7–9 This phenomenon is loosely referred to as “resistance” without a clear, consensus definition.10,11 In most instances, what is described as resistance is actually either hyporesponsiveness to therapy, which falls under platelet response variability, or patient non-adherence, which may or may not be obvious.12,13 There are many potential reasons for platelet response variability, including adherence, non-absorption, and genetic polymorphisms (Table 1).

True pharmacological resistance is probably uncommon and likely the result of genetic polymorphism. Some evidence suggests that variability in cytochrome P450-dependent enzyme activity due to genetic polymorphism may contribute to inter-patient variation in aspirin and clopidogrel response.14,15 ACS patients are likely to be taking multiple medications for co-morbid conditions, including statins and/or calcium-channel blockers, that are metabolized by cytochrome P3A4 (CYP 3A4). Non-dihydropyridines such as verapamil and diltiazen are known to inhibit CYP 3A4, and most statins, with the exception of pravastatin, compete with clopidogrel for binding to CYP 3A4; this could lead to reduced metabolism or clearance of one or both of the drugs involved.16 Conversely, a study conducted in healthy volunteers showed that St. John’s Wort amplified the effects of clopidogrel, turning non-responders into responders.17 Recently, the FDA reported that additional studies would be conducted to better characterize the impact of genetic factors and concomitant administration of other drugs on the efficacy of clopidogrel.18
Laboratory platelet aggregation tests, traditionally used to evaluate bleeding disorders, have recently been employed to correlate ex vivo results of antiplatelet therapy with clinical outcomes. However, using these inhibition of platelet activity (IPA) results is problematic and currently clinically non-interpretable, partly due to the lack of a standard test for IPA. For each given test, there is considerable variation among laboratories as the methodology is difficult to standardize. Further, results of these tests are temporally variable for any given patient. More importantly, there have been no data definitively linking IPA with clinical outcomes. In studies of patients presenting to the ED with acute chest pain or ACS, the success of platelet function testing in predicting the severity of MI or other adverse cardiac events has been variable.19,20 In particular, results of IPA tests suggest ‘resistance’ upwards of 35%; in reality, however, only 8%–10% of patients show clinical signs of hyporesponsiveness or resistance.21 Clearly, platelet response variability to antiplatelet therapy is a controversial and widely debated topic that requires more research to discern its true clinical impact and whether any practice changes are necessary. Such changes are likely to occur first in the chronic management of coronary artery disease, but at some point in the future may impact ED decision-making as well.
Loading Dose
Rapid inhibition of platelet aggregation is often accomplished by administering a loading dose of an antiplatelet agent. As shown in the CURE,8,22 CREDO,7and CLARITY23,24 trials, as well as a meta-analysis thereof ,24 addition of a 300-mg clopidogrel loading dose resulted in significant relative risk reductions in endpoints among all ACS patients, regardless of their intervention strategy (Table 2). The optimal loading dose of clopidogrel necessary to safely achieve rapid platelet inhibition has been an area of investigation. Compared to the standard 300-mg loading dose, a 600-mg dose has been shown to inhibit platelet aggregation more rapidly, reducing the time required to achieve maximum platelet inhibition from six to two hours.25–31
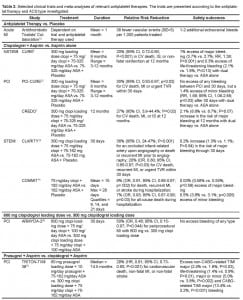
Although a higher clopidogrel loading dose more rapidly inhibits platelet aggregation, it is unclear whether this translates into improved clinical outcomes. In the ARMYDA-2 study of UA/NSTEMI patients undergoing PCI, a 600-mg clopidogrel loading dose reduced the risk of periprocedural events by 50% compared to a 300-mg loading dose without increasing the risk of bleeding (Table 2).30 In the CLEAR PLATELETS and ISAR-REACT 2 studies, addition of a GPI following a 600 mg clopidogrel loading dose further reduced the risk of adverse events and myocardial necrosis without significantly increasing the risk of bleeding.9,25 However, the added clinical benefits relative to the safety (bleeding risk) of higher loading doses remain to be fully established. This is being evaluated in the ongoing CURRENT OASIS-7 trial.
Current U.S. guidelines reflect the uncertainty of the optimal clopidogrel loading dose. Both the PCI and UA/NSTEMI guidelines specifically mention this uncertainty.2,32 The UA/NSTEMI guidelines don’t make a specific recommendation, while the PCI guidelines recommend a 600-mg loading dose.2,32 The STEMI guidelines maintain a recommendation of 300 mg for patients receiving fibrinolysis or no reperfusion.33
Loading doses of the recently approved antiplatelet agent, prasugrel, have also been assessed. In PRINCIPLE, a 60-mg prasugrel loading dose resulted in greater platelet inhibition compared to a 600-mg clopidogrel loading dose as early as 30 minutes after intake.34 Although PRINCIPLE was not powered to detect clinical outcomes, hemorrhagic adverse events were more common in patients taking prasugrel. The excess bleeding associated with prasugrel was more pronounced in TRITON, in which efficacy and safety were compared in PCI patients receiving prasugrel or clopidogrel (standard 300-mg dose); in this study, all classes of TIMI bleeding were significantly greater in patients taking prasugrel.35 In light of these findings, the U.S. prescribing information for prasugrel includes a black box warning highlighting its associated bleeding risks. Specifically, prasugrel is contraindicated in patients with active pathological bleeding or a history of stroke or TIA, should not be given to patients likely to undergo CABG, and is generally not recommended for patients aged ≥75 years.
CABG
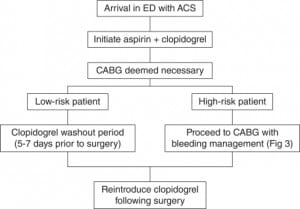
In ACS patients who undergo CABG, the addition of clopidogrel to aspirin increases bleeding risk if surgery is performed within five days after discontinuation.8 A dilemma thus arises, as it is difficult to predict prior to diagnostic angiography which patients will require urgent, early CABG.8,36 The EP can take one of two approaches to starting clopidogrel: 1) Initiate clopidogrel in the ED in all high-risk UA/NSTEMI patients, with a view to withdraw before emergency CABG or five to seven days before semi-elective or elective CABG; or 2) defer clopidogrel treatment until after angiography, therefore avoiding treatment in patients who require emergency CABG. The first strategy, recommended by the European Society of Cardiology (ESC),37 offers the advantages of reducing early ischemic events (relative risk reduction of 20%) and optimal timing for pre-PCI administration, but at the cost of increased peri-operative bleeding for patients who undergo early CABG.36,38 The second strategy offers the advantage of avoiding excess bleeding during early CABG, but at the cost of ischemic events and loss of pre-treatment benefit in PCI patients.8,39
It is important to remember the CRUSADE data, where only 12% of UA/NSTEMI patients underwent CABG during their index hospitalization.40 Other studies estimate rates of CABG between 8% and 25% during index hospitalization.8,41–44 Emergency CABG rates are seemingly lower, from 0.3% to 0.6%.45 Since the majority of patients are suitable for PCI or medical management, most high-risk ACS patients would thus benefit from early dual antiplatelet inhibition. Furthermore, among patients in CURE who underwent CABG, lower ischemic event rates were observed with clopidogrel treatment before CABG.36 Taking these considerations into account, it has been suggested that most patients requiring CABG will benefit from initiating clopidogrel and aspirin on admission (i.e. in the ED) and then stopping clopidogrel five days before surgery to minimize bleeding risk (Figure 2).36,46 Even if urgent CABG is required, evidence indicates that an experienced surgeon can perform CABG within five days of clopidogrel washout via judicious use of a bleeding management algorithm.47 One study found that CABG performed within five days of clopidogrel washout resulted in postoperative mortality rates similar to patients who were not exposed to clopidogrel within five days before CABG.47
Patients with hemodynamic instability (cardiogenic shock), mechanical complications (acute mitral regurgitation), diabetes, impaired left ventricular function, concomitant vascular disease, and multivessel disease are at higher risk for urgent CABG.2,48 As shown in the Bypass Angioplasty Revascularization Investigation study of patients with diabetes,49 such patients may have improved survival with CABG compared to PCI. It would therefore be prudent to withhold clopidogrel in these patients. For patients for whom clopidogrel pre-treatment is withheld pending angiography, data from ISAR-REACT 2 suggest that GPIs be administered upstream of the catheterization lab in troponin-positive patients 9
Based on an analysis of NSTEMI patients from the TACTICS-TIMI-18 trials, Sadanandan et al.50 developed a predictive risk score to identify patients who are likely to require CABG during index hospitalization. Mehta et al.51 have also developed a multivariate model, based on CRUSADE data, identifying 13 presenting clinical characteristics significantly associated with undergoing CABG during initial hospital stay. However, identification in the ED of patients likely to need urgent CABG remains problematic as these prediction scores are often unreliable prior to diagnostic angiography. Because of the difficulty in predicting which ACS patients will require emergency CABG, it is essential that emergency physicians, cardiologists, cardiovascular surgeons, and hospitalists develop clear, institution-specific indications for clopidogrel and GPI administration. Such collaboration decreases reliance on personal preferences and empowers emergency physicians to initiate care and gain ischemia-related reductions while simultaneously maximizing patient safety.
Safety Considerations
The risk of bleeding is the most important safety consideration when initiating antiplatelet therapy. This risk must be weighed against observed clinical benefits in all ACS patients. As might be expected based on higher levels of platelet inhibition, bleeding risk is increased by combining antiplatelet agents (Table 2). Among ACS patients, adding clopidogrel to aspirin is associated with an absolute 0.2% to 1.0% increase in major bleeding.52 However, the statistical significance of this increased bleeding varied among the trials. This may be partly due to the definition used to classify bleeding events. For example, the excess bleeding in CURE was significant when the OASIS scale was used but insignificant using the TIMI and GUSTO scales.8 Importantly, even using the stringent OASIS scale, life-threatening bleeding was not significantly greater among dual aspirin and clopidogrel recipients in CURE.
In contrast to the well-established safety and efficacy data of dual treatment with aspirin and clopidogrel, data related to dual therapy with aspirin and prasugrel are only emerging, and their overall clinical significance is yet unknown. In TRITON, the superior efficacy of prasugrel and aspirin was accompanied by significant excesses of non-CABG-related TIMI major and minor bleeding, life-threatening and fatal bleeding, bleeding requiring transfusion, and CABG-related TIMI major bleeding (Table 2).35 The risk of bleeding was particularly prominent in patients with a history of stroke or TIA and those aged ≥75 years or with a body weight <60 kg. The resultant unfavorable net benefit in these patient subgroups led to the inclusion of the aforementioned black box warning in the prasugrel prescribing information. Additional data are required to fully establish the net benefit of dual therapy with aspirin and prasugrel.
The safety profiles of AZD6140 and cangrelor, two emerging antiplatelet agents which are reversible inhibitors of the P2Y12 receptor, also remain to be determined. In early trials, AZD6140 induced hypotension and dyspnea, potentially problematic side effects that may mimic symptoms of recurrent atherothrombotic events.53 Additionally, AZD610 has a higher IPA than clopidogrel and may lead to an increased risk of bleeding in certain patient populations.53 While cangrelor did not show significant excess bleeding in early trials,54,55 more data are needed.
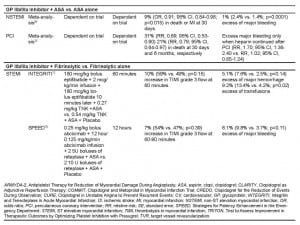
GPI inhibition is associated with a small, significant increased incidence of bleeding, most commonly at the vascular access site (Table 2). In a meta-analysis, GPI use in UA/NSTEMI patients was associated with a significant excess of major bleeding complications (2.4% vs. 1.4%, p<0.0001), though intracranial bleeding was not increased significantly.56 It is important to note, however, that this increased bleeding risk was offset by significant reductions in death and MI, particularly in high-risk patients. Among STEMI patients treated by fibrinolysis, GPI therapy is not associated with a net clinical benefit as major bleeding is significantly increased in the absence of any mortality reductions (Table 3).57,58 In contrast, the ADMIRAL59 and CADILLAC60 studies showed that benefits from upstream GPI therapy in PCI patients were not compromised by any important increased bleeding risk. In the ACUITY trial,61 which investigated an early invasive strategy in UA/NSTEMI patients, the combination of bivalirudin, a direct thrombin inhibitor, with GPI therapy was associated with comparable clinical outcomes and a lower bleeding risk compared with heparin plus GPI therapy.
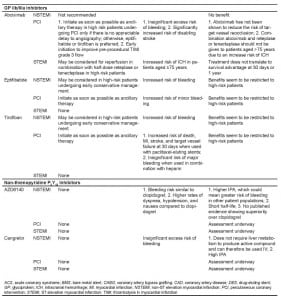
Overall, a large body of evidence supports an acceptable safety profile for dual antiplatelet therapy with aspirin and clopidogrel in all ACS patients, a differential safety profile with GPIs, and an unclear safety profile for the novel agents prasugrel, AZD6140, and cangrelor.
Evidence-based, practical solutions for antiplatelet therapy
The 2007 ACC/AHA guidelines recommend that all ACS patients receive aspirin and clopidogrel (loading dose followed by a maintenance dose) as soon as possible regardless of reperfusion strategy (Table 3).2 Antiplatelet therapy should not be withheld prior to catheterization. If the patient has already received clopidogrel and elective CABG is deemed necessary, clopidogrel should be discontinued for five to seven days prior to surgery in order to balance antiplatelet efficacy with bleeding risk. The addition of a GPI depends on the management strategy and risk level of the patient.2 In UA/NSTEMI patients, a GPI should be given upstream of, or immediately prior to, PCI. The guidelines advocate GPI administration as early as possible in STEMI patients undergoing PCI,32,33,62,63 which supports initiating treatment in the ED.
Results from various studies supporting these guidelines are of particular interest to EPs. One example is the finding from CURE supporting early initiation of dual antiplatelet inhibition in UA/NSTEMI patients. A statistically significant benefit of dual antiplatelet therapy over aspirin alone in reducing ischemic events was evident as early as 24 hours after randomization, with the curves separating qualitatively at 12 hours (Figure 3).8Other evidence supports pretreatment with a clopidogrel loading dose prior to PCI.7,22Among UA/NSTEMI patients in CREDO and those undergoing PCI in CURE, adding a clopidogrel loading dose resulted in significant relative risk reductions in endpoints (Table 2).22 The CLARITY and COMMIT trials, which evaluated clopidogrel treatment in conjunction with fibrinolytics, aspirin and heparin in STEMI patients, reported significant cardiovascular event reductions in patients pretreated with clopidogrel (Table 2).23
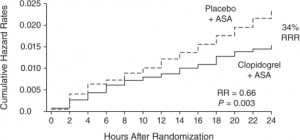
CREDO also raised the issue of timing in UA/NSTEMI patients, suggesting that longer intervals between dosing and PCI might show greater benefit, ostensibly by allowing clopidogrel to achieve maximum platelet inhibition.7 It should be emphasized that one of the most compelling reasons to initiate clopidogrel therapy as early as possible is to decrease periprocedural ischemia.30 In the ARMYDA-2 trial, patients were randomized to loading doses of either 600-mg or the conventional 300-mg 4–8 hours prior to angiography.30 The primary endpoint was the 30-day occurrence of death, MI, or target vessel revascularization. The primary endpoint occurred in 4% of patients in the high-loading dose group versus 12% of those in the conventional-loading dose group (P=0.041) and was due entirely to periprocedural MI. However, in a small study of patients with ACS undergoing stent implantation, no difference was found with three days of clopidogrel pretreatment compared with standard post-procedural treatment in troponin I or creatinine kinase-MB serum levels up to 24 hours after PCI.64
Although it is important for EPs to appreciate the added periprocedural protection that upstream administration of advanced antiplatelet therapy affords their ACS patients, the ideal timing of clopidogrel initiation is uncertain. The guidelines recommend administration as soon as possible, and as the time from administration to PCI increases, so does the periprocedural protection. The ARMYDA-2 trial gave a loading dose 4–6 hours prior to PCI, but the EP is often dealing with a 90-minute treatment window, making the ARMYDA-2 results relevant primarily to those ACS patients not going emergently for PCI.
Similarly, the optimal timing for GPI initiation prior to PCI is unclear.4 In PURSUIT, the reduction in death and MI was inversely associated with the time from symptom onset to GPI initiation;4 however, data from PRISM and NRMI 4 suggest no difference in outcomes as long as the drug was initiated within 24 hours of symptom onset.65,66 In the absence of clear evidence, ED physicians must carefully weigh each individual patient’s characteristics and clinical symptoms when making a decision concerning GPI initiation.
The adjunctive use of GPIs in STEMI patients depends on the planned treatment course. In the setting of PCI, the ADMIRAL study showed a significant reduction in death/reinfarction/urgent revascularization at both 30 days and six months when adjunctive abciximab was administered prior to the procedure.59 The CADILLAC study confirmed the protective effect of abciximab in the short term (35% relative risk reduction for death, MI, target vessel revascularization or stroke at 30 days), although this benefit was no longer apparent at one year.60 Data from ADMIRAL and CADILLAC, conducted in the PCI setting, show that the protective benefits of pre-procedural abciximab are not compromised by any important increase in bleeding risk. In contrast, the ASSENT-3 and GUSTO V trials demonstrated that the combination of a GPI with half-dose thrombolytic reduced ischemic events but increased bleeding; furthermore, there was no short- or long-term survival benefit.67–71 These findings suggest that adding a GPI is not justified during fibrinolytic treatment of STEMI.
CONCLUSION
The EP’s role in treating ACS patients is to provide rapid, accurate diagnosis and institute timely, risk-directed treatment. Increasing the potency of platelet inhibition by adding a thienopyridine or GPI to standard therapy early in the treatment course improves protection against ischemic and periprocedural events but must be balanced against any unjustifiable increase in bleeding risk. Clopidogrel plus aspirin is a safe and effective therapy recommended by national guidelines for use in ACS patients, regardless of treatment strategy (Table 4).
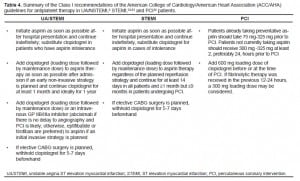
Choice and timing of antiplatelet therapy in the ED must also take into consideration future interventions the patients may receive during their hospital course. In addition to standard aspirin therapy, early initiation of clopidogrel in the ED is often justified in ACS patients, regardless of their subsequent treatment strategy (medical or interventional). However, care should be taken regarding patients who are highly likely to require early CABG. Early administration of a GPI is also often justified in ACS patients in the PCI setting and in high-risk UA/NSTEMI patients in whom medical management is planned. Results from the ASSENT-3 and GUSTO-V trials do not support a favorable balance of benefit over bleeding risk for GP inhibition in STEMI patients undergoing fibrinolysis. This finding illustrates the complexity of balancing increased antiplatelet potency with bleeding risk.
Emerging investigational antiplatelet agents may show promise in ACS treatment; however, use of these agents should be approached with caution. Although touting increased IPA, it should be recalled that traditionally this has been a measure of bleeding, not potency. Therefore, long-term risks for bleeding and compliance may become issues. As the science of emergency cardiology care continues to mature and evolve at a rapid pace, continuous, evidence-based, multidisciplinary collaboration is paramount for delivering optimal and safe care for ACS patients. Given the variability in treatment preferences and awareness of guideline recommendations, there is an important need for developing institutional protocols and order sheets in order to improve adherence to treatment guidelines.
Footnotes
Editorial support in preparation of this manuscript was funded by the Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership. The authors did not receive any compensation for this work.
Supervising Section Editor: J. Christian Fox, MD
Submission history: Submitted August 24, 2008; Revision Received January 25, 2009; Accepted January 26, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: David E. Slattery, MD, Department of Emergency Medicine, University of Nevada School of Medicine, 901 Rancho Lane, Suite #135, Las Vegas, NV 89106
Email: dslatts@mac.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. Editorial support in preparation of this manuscript was funded by the Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership. The authors did not receive any compensation for this work.
REFERENCES
1. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA.2000;284:835–42. [PubMed]
2. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation.2007;116:e148–304. [PubMed]
3. Cannon CP. Risk stratification and the management of non-ST-segment elevation acute coronary syndromes: The role of antiplatelet therapy. Crit Pathw Cardiol. 2004;3:83–86. [PubMed]
4. Fesmire FM, Decker WW, Diercks DB, et al. Clinical policy: critical issues in the evaluation and management of adult patients with non-ST-segment elevation acute coronary syndromes. Ann Emerg Med. 2006;48:270–301. [PubMed]
5. Mehta RH, Roe MT, Chen AY, et al. Recent trends in the care of patients with non-ST-segment elevation acute coronary syndromes: insights from the CRUSADE initiative.Arch Intern Med. 2006;166:2027–34. [PubMed]
6. Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101:570–80. [PubMed]
7. Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial.JAMA. 2002;288:2411–20. [PubMed]
8. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med.2001;345:494–502. [PubMed]
9. Kastrati A, Mehilli J, Neumann FJ, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006;295:1531–8. [PubMed]
10. Barsky AA, Arora RR. Clopidogrel resistance: myth or reality? J Cardiovasc Pharmacol Ther. 2006;11:47–53. [PubMed]
11. Wiviott SD, Antman EM. Clopidogrel resistance: a new chapter in a fast-moving story.Circulation. 2004;109:3064–7. [PubMed]
12. Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance.Arterioscler Thromb Vasc Biol. 2004;24:1980–7. [PubMed]
13. Seyfert UT, Haubelt H, Vogt A, et al. Variables influencing multiplate whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets. 2007;18:199–206. [PubMed]
14. Lau WC, Gurbel PA, Watkins PB, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation.2004;109:166–71. [PubMed]
15. Suh JW, Koo BK, Zhang SY, et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. Cmaj.2006;174:1715–22. [PMC free article] [PubMed]
16. Bottorff M, Hansten P. Long-term safety of hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitors: the role of metabolism-monograph for physicians. Arch Intern Med. 2000;160:2273–80. [PubMed]
17. Lau WC, Carville DG, Guyer KE, et al. St. John’s wort enhances the platelet inhibitory effect of clopidogrel in clopidogrel “resistant” healthy volunteers. J Am Coll Cardiol.2005;45:382A.
18. US Food and Drug Administration Center for Drug Evaluation and Research Bethesda, MD: US Dept of Health and Human Services; 2009. Early communication about an ongoing safety review of clopidogrel bisulfate (marketed as Plavix)
19. Frossard M, Fuchs I, Leitner JM, et al. Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation. 2004;110:1392–7. [PubMed]
20. Selvaraj CL, Van De Graaff EJ, Campbell CL, et al. Point-of-care determination of baseline platelet function as a predictor of clinical outcomes in patients who present to the emergency department with chest pain. J Thromb Thrombolysis. 2004;18:109–15.[PubMed]
21. Cattaneo M. Resistance to antiplatelet drugs: molecular mechanisms and laboratory detection. J Thromb Haemost. 2007;5(Suppl 1):230–7. [PubMed]
22. Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–33. [PubMed]
23. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med.2005;352:1179–89. [PubMed]
24. Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005;294:1224–32. [PubMed]
25. Gurbel PA, Bliden KP, Zaman KA, et al. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation. 2005;111:1153–9.[PubMed]
26. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. High clopidogrel loading dose during coronary stenting: effects on drug response and interindividual variability. Eur Heart J. 2004;25:1903–10. [PubMed]
27. Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol. 2006;48:1339–45.[PubMed]
28. Longstreth KL, Wertz JR. High-dose clopidogrel loading in percutaneous coronary intervention. Ann Pharmacother. 2005;39:918–22. [PubMed]
29. Muller I, Seyfarth M, Rudiger S, et al. Effect of a high loading dose of clopidogrel on platelet function in patients undergoing coronary stent placement. Heart. 2001;85:92–3. [PMC free article] [PubMed]
30. Patti G, Colonna G, Pasceri V, et al. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2005;111:2099–106.[PubMed]
31. von Beckerath N, Taubert D, Pogatsa-Murray G, et al. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation. 2005;112:2946–50.[PubMed]
32. King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:172–209. [PubMed]
33. Antman EM, Hand M, Armstrong PW, et al. 2007 Focused update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–47. [PubMed]
34. Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation.2007;116:2923–32. [PubMed]
35. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. [PubMed]
36. Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004;110:1202–8. [PubMed]
37. Bassand JP, Hamm CW, Ardissino D, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–660. [PubMed]
38. Hongo RH, Ley J, Dick SE, et al. The effect of clopidogrel in combination with aspirin when given before coronary artery bypass grafting. J Am Coll Cardiol. 2002;40:231–7.[PubMed]
39. Mehta RH, Roe MT, Mulgund J, et al. Acute clopidogrel use and outcomes in patients with non-ST-segment elevation acute coronary syndromes undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2006;48:281–6. [PubMed]
40. Duke Clinical Research Institute The CRUSADE national quality improvement initiative, 2006 4th quarter resultsAvailable at:http://www.crusadeqi.com/Main/HomePage.shtml Accessed April 27, 2008.
41. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. N Engl J Med. 1998;338:1488–97. [PubMed]
42. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med.1998;339:436–43. [PubMed]
43. Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–87. [PubMed]
44. Ferguson JJ, Califf RM, Antman EM, et al. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292:45–54. [PubMed]
45. Yang EH, Gumina RJ, Lennon RJ, et al. Emergency coronary artery bypass surgery for percutaneous coronary interventions: changes in the incidence, clinical characteristics, and indications from 1979 to 2003. J Am Coll Cardiol. 2005;46:2004–9.[PubMed]
46. Peters RJ. Is clopidogrel associated with poor outcome in patients with non-ST-segment elevation ACS after early CABG surgery? Nat Clin Pract Cardiovasc Med.2007;4:24–5. [PubMed]
47. Chen L, Bracey AW, Radovancevic R, et al. Clopidogrel and bleeding in patients undergoing elective coronary artery bypass grafting. J Thorac Cardiovasc Surg.2004;128:425–31. [PubMed]
48. Bavry AA, Lincoff AM. Is clopidogrel cardiovascular medicine’s double-edged sword?Circulation. 2006;113:1638–40. [PubMed]
49. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217–25. [PubMed]
50. Sadanandan S, Cannon CP, Gibson CM, et al. A risk score to estimate the likelihood of coronary artery bypass surgery during the index hospitalization among patients with unstable angina and non-ST-segment elevation myocardial infarction. J Am Coll Cardiol.2004;44:799–803. [PubMed]
51. Mehta RH, Chen AY, Pollack CV, Jr, et al. Challenges in predicting the need for coronary artery bypass grafting at presentation in patients with non-ST-segment elevation acute coronary syndromes. Am J Cardiol. 2006;98:624–7. [PubMed]
52. Eikelboom JW, Hirsh J. Bleeding and management of bleeding. Eur Heart J.2006;8:G38–45.
53. Husted S, Emanuelsson H, Heptinstall S, et al. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–47. [PubMed]
54. Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J. 2006;151:689 e1–689 e10. [PubMed]
55. Jacobsson F, Swahn E, Wallentin L, et al. Safety profile and tolerability of intravenous AR-C69931MX, a new antiplatelet drug, in unstable angina pectoris and non-Q-wave myocardial infarction. Clin Ther. 2002;24:752–65. [PubMed]
56. Boersma E, Harrington RA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials.Lancet. 2002;359:189–98. [PubMed]
57. Brindis RG, Fischer E, Besinque G, et al. Acute coronary syndromes clinical practice guidelines. Crit Pathw Cardiol. 2006;5:69–102. [PubMed]
58. De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA. 2005;293:1759–65. [PubMed]
59. Montalescot G, Barragan P, Wittenberg O, et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med.2001;344:1895–903. [PubMed]
60. Tcheng JE, Kandzari DE, Grines CL, et al. Benefits and risks of abciximab use in primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial.Circulation. 2003;108:1316–23. [PubMed]
61. Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–16. [PubMed]
62. Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–286. [PubMed]
63. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. [PubMed]
64. van der Heijden DJ, Westendorp IC, Riezebos RK, et al. Lack of efficacy of clopidogrel pre-treatment in the prevention of myocardial damage after elective stent implantation.J Am Coll Cardiol. 2004;44:20–4. [PubMed]
65. A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. N Engl J Med. 1998;338:1498–505. [PubMed]
66. Peterson ED, Pollack CV, Jr, Roe MT, et al. Early use of glycoprotein IIb/IIIa inhibitors in non-ST-elevation acute myocardial infarction: observations from the National Registry of Myocardial Infarction 4. J Am Coll Cardiol. 2003;42:45–53. [PubMed]
67. Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605–13. [PubMed]
68. Sinnaeve PR, Alexander JH, Bogaerts K, et al. Efficacy of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: one-year follow-up results of the Assessment of the Safety of a New Thrombolytic-3 (ASSENT-3) randomized trial in acute myocardial infarction. Am Heart J. 2004;147:993–8. [PubMed]
69. Topol EJ. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet. 2001;357:1905–14. [PubMed]
70. Lincoff AM, Califf RM, Van de Werf F, et al. Mortality at 1 year with combination platelet glycoprotein IIb/IIIa inhibition and reduced-dose fibrinolytic therapy vs conventional fibrinolytic therapy for acute myocardial infarction: GUSTO V randomized trial. JAMA. 2002;288:2130–5. [PubMed]
71. Gurm HS, Lincoff AM, Lee D, et al. Outcome of acute ST-segment elevation myocardial infarction in diabetics treated with fibrinolytic or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: lessons from the GUSTO V trial. J Am Coll Cardiol. 2004;43:542–8. [PubMed]
72. Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity.Circulation. 2003;107:2908–13. [PubMed]
73. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ.2002;324:71–86. [PMC free article] [PubMed]
74. Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet.2005;366:1607–21. [PubMed]
75. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2007
76. Karvouni E, Katritsis DG, Ioannidis JP. Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. J Am Coll Cardiol. 2003;41:26–32. [PubMed]
77. Giugliano RP, Roe MT, Harrington RA, et al. Combination reperfusion therapy with eptifibatide and reduced-dose tenecteplase for ST-elevation myocardial infarction: results of the integrilin and tenecteplase in acute myocardial infarction (INTEGRITI) Phase II Angiographic Trial. J Am Coll Cardiol. 2003;41:1251–60. [PubMed]
78. Trial of abciximab with and without low-dose reteplase for acute myocardial infarction. Strategies for Patency Enhancement in the Emergency Department (SPEED) Group. Circulation. 2000;101:2788–94. [PubMed]
79. Grines CL, Bonow RO, Casey DE, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115:813–8. [PubMed]
80. Yusuf S, Mehta SR, Zhao F, et al. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation. 2003;107:966. [PubMed]


