| Author | Affiliation |
|---|---|
| James H. Moak, MD | University of Virginia Health System, Department of Emergency Medicine, Charlottesville, Virginia |
| Aaron J. Vaughan, MD | University of Virginia Health System, Department of Family Medicine, Charlottesville, Virginia |
| Benjamin A. Silverberg, MD | University of Virginia Health System, Department of Family Medicine, Charlottesville, Virginia |
ABSTRACT
Children presenting to the emergency department with hip pain and fever are at risk for significant morbidity due to septic arthritis. Distinguishing between septic arthritis and other causes of hip pain may be challenging. Sonographic visualization of the hip with real-time ultrasound-guided arthrocentesis may allow faster differentiation between etiologies, hastening definitive therapy and improving analgesia. This report describes the use of hip sonography in a case of Lyme arthritis. The authors review the medical literature in support of bedside hip sonography and discuss how to perform ultrasound-guided hip arthrocentesis. Clinical findings in septic and Lyme arthritis are also described.
VIDEO NOT FOUND ON E-SCHOLARSHIP
INTRODUCTION
Distinguishing between septic arthritis and other causes of nontraumatic hip pain can be difficult in pediatric patients. The diagnostic evaluation typically involves arthrocentesis of the affected joint by a consultant in the radiology suite or operating room. Depending upon the availability of hospital resources and personnel, delays may occur before synovial fluid is obtained and effective treatment initiated. We present a case in which bedside ultrasound-guided hip arthrocentesis by the emergency physician expedited empiric treatment and disposition of a febrile patient with hip pain.
CASE
A previously healthy 7-year-old boy presented to the emergency department (ED) with a complaint of right lower extremity pain and fever. The preceding day his parents had noticed that he was limping upon returning home from school. The pain progressed that evening until he could no longer bear weight on his right leg. Overnight he developed a fever. The child reported no history of trauma. His parents stated that 2 days before presentation he had complained of contralateral leg pain, which had since resolved. On further questioning, his parents also reported that he had experienced left shoulder pain 3 days previously, which resolved within 36 hours. Neither the child nor his parents were aware of any recent tick exposures, but they did note that he had had tick bites several months prior during the summer. He was described as a very active child who enjoys playing outdoors. Other than a recent trip to Europe, there was no history of foreign travel. There were no known sick contacts. Review of systems was positive for fatigue, but negative for chills, weight loss, nausea, vomiting, diarrhea, abdominal pain, cough, congestion, rhinorrhea, sore throat, and urinary symptoms.
Physical examination revealed a pleasant, well-developed boy who appeared uncomfortable and localized his pain to the right anterior mid-thigh. His temperature was 38.0°C; heart rate, 132 beats/minute; respiratory rate, 24 breaths/minute; blood pressure, 106/54 mmHg; and O2 saturation, 99% on room air. He was unable to bear weight on the right leg owing to pain. He had no tenderness to palpation at the right hip or knee, but had pain with passive internal and external rotation of the affected hip. He could flex and extend the right knee without difficulty. He had no appreciable edema at the right knee or hip but did have an effusion at the asymptomatic left knee. There was no erythema or calor present in either lower extremity. A thorough inspection of the skin and scalp revealed no rash or signs of insect bites. The remainder of the physical examination was unremarkable.
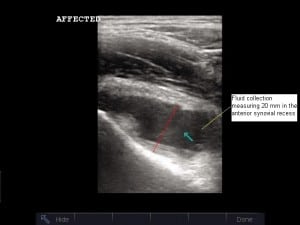
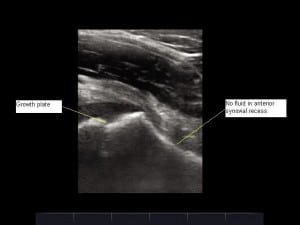
Serologic studies revealed a white blood cell (WBC) count of 8,900/μL, erythrocyte sedimentation rate (ESR) of 53 mm/h, and C-reactive protein (CRP) of 5.7 mg/dL. Blood cultures and Lyme titers were obtained. Radiographs showed no abnormality at either hip, a small to moderate effusion of the right knee, and a moderate effusion of the left knee. Bedside ultrasonography was performed, revealing a hypoechoic fluid collection at the right hip (Figure 1) measuring 20 mm in the anterior synovial recess, which is the space between the anterior cortex of the femoral neck and the posterior surface of the joint capsule underlying the iliopsoas muscle. Normally, a fluid collection in this location, if present at all, should be no greater than 7.7 mm for a child of this age.1 Sonography of the left hip was normal (Figure 2).
After applying topical anesthetic cream and injecting subcutaneous local anesthetic, an ultrasound-guided arthrocentesis of the right hip was performed in the ED under sterile conditions (video of procedure available online). Seven milliliters of turbid, yellow fluid was aspirated and sent for analysis. Repeated sonographic imaging of the right hip revealed a marked reduction in size of the effusion. After arthrocentesis, ceftriaxone was promptly administered intravenously, and the orthopedic service was consulted.
The synovial fluid had 109,495 white cells/mm3 (95% neutrophils). The gram stain results showed no organisms. When examined by the orthopedist approximately an hour after arthrocentesis, the patient had significant improvement in his pain and was able to bear weight on the affected leg. A second arthrocentesis, this time of the left knee, was performed by the orthopedic consultant that yielded 82,887 white cells/mm3 (92% neutrophils) and a negative gram stain result. In view of these findings, Lyme disease was believed to be the likely cause of the patient’s illness. The patient was admitted to the family practice service, and ceftriaxone therapy was continued. The patient’s symptoms improved in hospital, and after 3 days he was discharged and given a 4-week course of amoxicillin for presumptive Lyme arthritis. Ultimately, blood and synovial fluid cultures revealed no growth. Lyme studies, which were pending at the time of discharge, yielded positive results 3 days later by Western Blot analysis for IgG and IgM.
DISCUSSION
The differential diagnosis for a child with acute hip pain can be divided into disorders that are usually apparent on plain radiography (eg, fracture, neoplasm, slipped capital femoral epiphysis, and Perthes disease) and ones that are not (eg, transient synovitis, Lyme disease, and septic arthritis). If plain radiographs yield negative findings, early identification or exclusion of septic arthritis is critical. Without prompt treatment, septic arthritis can lead to osteonecrosis, osteomyelitis, epiphyseal damage, and systemic sepsis.2–6
Ultrasonography has long been used to evaluate hip disorders and is considered the preferred modality for diagnosing hip effusions in children.4,7 In 1980, Graf8 described the use of ultrasound to evaluate congenital hip dislocations. Ultrasound-guided arthrocentesis of the hip was later described by Hill and colleagues9 using a static approach, and by Mayakawa et al10 using real-time sonography. Only 3 prior reports of emergency physician–performed hip arthrocentesis have been published.11–13 Smith11 first reported using this technique in the ED in a 47-year-old male found to have pseudogout. Freeman et al12 described 4 adult patients who underwent ultrasound-guided arthrocentesis in the ED; 3 were found to have a nonseptic reactive monoarthropathy and 1 had avascular necrosis. Finally, Tsung and Blaivas13 performed bedside ultrasound-guided arthrocentesis in 2 pediatric patients, of whom 1 was diagnosed with transient synovitis and 1 with septic arthritis. A review of the medical literature reveals no prior report of ultrasound-guided arthrocentesis in a patient with Lyme arthritis.
This case illustrates the potential for accelerating synovial fluid analysis and antibiotic therapy in suspected septic arthritis with bedside, ultrasound-guided arthrocentesis. At our institution, hip arthrocentesis is typically performed by an orthopedist in the radiology suite or operating room, using fluoroscopic guidance. Anecdotally, consulting orthopedists often prefer that antibiotics be withheld until synovial fluid can be obtained. Depending upon the availability of resources and personnel, significant delays may occur before arthrocentesis is performed. In the case presented, synovial fluid analysis and antibiotic therapy occurred promptly after initial radiographs and serologic studies.
Prior investigators have shown that hip sonography is an easily learned technique. Vieira and Levy14found that pediatric emergency physicians with minimal training in ultrasonography could identify hip effusions in children, with a sensitivity and specificity of 80% and 98%, respectively, after only 10 training examinations. A high-frequency, linear array transducer is placed over the proximal femur obliquely from inferolateral to superomedial, in plane with the long axis of the femoral neck. The probe indicator is directed toward the umbilicus. (As long as the screen indicator, or dot, is positioned on the left side of the screen, the femoral head will be seen on the left-hand side of the image regardless of which hip is being evaluated.) If present, an effusion will be visualized in the anterior synovial recess overlying the anterior surface of the femoral neck (Figure 1). Of note, Rohrschneider et al15 found that 12% of asymptomatic children will have a thin layer of synovial fluid in this space. Tien and colleagues1 have defined the normal range in millimeters for such a fluid collection as less than or equal to 6.52 mm + 0.013 × (age in months). Thus, the 93-month-old child in this case could have a fluid collection up to 7.7 mm wide in the anterior synovial recess in the absence of pathology. This measurement should be made from the apex of the concavity of the femoral neck (ie, the deepest point in the anterior synovial recess) to the posterior surface of the anterior joint capsule, which lies just posterior to the iliopsoas muscle. Tien et al1 additionally reported that an effusion 1.46 mm wider than on the contralateral side is also abnormal.
When performing ultrasound-guided hip arthrocentesis, the operator must take care to avoid the femoral artery and vein, which are visualized by scanning more medially. Using an in-plane approach, the needle is directed into the anterior synovial recess with real-time visualization of the long axis of the needle. We recommend an approach from the inferolateral side of the transducer. In a case such as this one, involving a right-handed operator approaching the patient’s right hip, we suggest seating oneself next to the patient’s right flank facing the patient’s feet, with the ultrasound machine adjacent to the patient’s right knee. For the left hip, a right-handed operator may be seated next to the patient’s left knee with the machine adjacent to the patient’s left shoulder. Sterile technique should be maintained throughout the procedure. A local anesthetic is advisable, though younger patients may require sedation in addition.
This case of Lyme arthritis was unusual in that the predominant joint involved was the hip. Lyme arthritis, the most common late manifestation of infection with Borrelia burgdorferi,16,17 is characterized by intermittent, asymmetric, monoarthritic or polyarthritic attacks of the large joints, most commonly the knee.18 Distinguishing between Lyme and septic arthritis can be challenging. Most children with Lyme arthritis present without erythema migrans18 and do not recall a tick bite.18,19 Although involvement of the knee, absence of fever, and a low CRP make Lyme disease more likely, the discriminatory value of these findings is inadequate for excluding septic arthritis.19 Our patient’s synovial fluid WBC count, greater than 100,000/mm3, was much higher than the mean value of 46,000/mm3 reported by Thompson et al19 in patients with Lyme arthritis. Synovial WBC, however, is known to have a wide range in patients with Lyme disease.19 The synovial fluid gram stain test, which has limited sensitivity for septic arthritis of only 29% to 50%,20 yielded a negative finding. To help differentiate between septic arthritis and transient synovitis, Kocher and colleagues21 validated a clinical decision rule consisting of a history of fever, non–weight-bearing status, ESR >40 mm/h, and WBC count >12,000 cells/mm3, each of which makes septic arthritis more likely. The presence of 3 of these predictors, as our patient had, yields a positive predictive value of 73% for septic arthritis.21Arthrocentesis and early antibiotic administration were, therefore, critical steps in this patient’s management. In addition to accelerating the diagnostic work-up and presumptive treatment, we found that bedside ultrasound-guided hip arthrocentesis had an additional benefit in alleviating our patient’s pain in the ED. Pain relief from joint decompression has been highlighted by others as an advantage of this procedure.6
CONCLUSION
This case of acute hip pain in a patient ultimately found to have Lyme arthritis demonstrates the potential for bedside ultrasound-guided arthrocentesis to accelerate diagnostic testing and empiric antibiotic therapy in cases of suspected septic arthritis. Earlier, more effective analgesia is an additional benefit. Bedside hip sonography and arthrocentesis appear to be useful skills in the ED setting. Further study is needed to determine the amount of training required for proficiency.
Footnotes
Supervising Section Editor: Seric S. Cusick, MD
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
Address for correspondence
James H. Moak, MD
University of Virginia Health System, Department of Emergency Medicine
PO Box 800699, Charlottesville, VA 22908-0699
E-mail: james.moak@virginia.edu
REFERENCES
1. Tien YC, Yang CY, Chih HW. The normal width of anterior hip synovial recess in children. J Pediatr Orthop. 2000;20:264–266. [PubMed]
2. Sultan J, Hughes PJ. Septic arthritis or transient synovitis of the hip in children: the value of clinical prediction algorithms. J Bone Joint Surg Br. 2010;92:1289–1293. [PubMed]
3. Bennett OM, Namnyak SS. Acute septic arthritis of the hip joint in infancy and childhood. Clin Orthop Relat Res. 1992;281:123–132. [PubMed]
4. Kang SN, Sanghera T, Mangwani J, et al. The management of septic arthritis in children: systematic review of the English language literature. J Bone Joint Surg Br. 2009;91:1127–1133. [PubMed]
5. Wingstrand H, Egund N, Lidgren L, et al. Sonography in septic arthritis of the hip in the child: report of four cases. J Pediatr Orthop. 1987;7:206–209. [PubMed]
6. Fink AM, Berman L, Edwards D, et al. The irritable hip: immediate ultrasound guided aspiration and prevention of hospital admission. Arch Dis Child. Arch Dis Child. 1995;1995;7272:3–4. 110–113.[discussion in.
7. Zamzam MM. The role of ultrasound in differentiating septic arthritis from transient synovitis of the hip in children. J Pediatr Orthop B. 2006;15:418–422. [PubMed]
8. Graf R. The diagnosis of congenital hip-joint dislocation by the ultrasonic Combound treatment. Arch Orthop Trauma Surg. 1980;97:117–133. [PubMed]
9. Hill SA, MacLarnon JC, Nag D. Ultrasound-guided aspiration for transient synovitis of the hip. J Bone Joint Surg Br. 1990;72:852–853. [PubMed]
10. Mayekawa DS, Ralls PW, et al. Sonographically guided arthrocentesis of the hip. J Ultrasound Med.1989;8:665–667. [PubMed]
11. Smith SW. Emergency physician-performed ultrasonography-guided hip arthrocentesis. Acad Emerg Med. 1999;6:84–86. [PubMed]
12. Freeman K, Dewitz A, Baker WE. Ultrasound-guided hip arthrocentesis in the ED. Am J Emerg Med. 2007;25:80–86. [PubMed]
13. Tsung JW, Blaivas M. Emergency department diagnosis of pediatric hip effusion and guided arthrocentesis using point-of-care ultrasound. J Emerg Med. 2008;35:393–399. [PubMed]
14. Vieira RL, Levy JA. Bedside ultrasonography to identify hip effusions in pediatric patients. Ann Emerg Med. 2010;55:284–289. [PubMed]
15. Rohrschneider WK, Fuchs G, Troger J. Ultrasonographic evaluation of the anterior recess in the normal hip: a prospective study on 166 asymptomatic children. Pediatr Radiol. 1996;26:629–634.[PubMed]
16. Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. [PubMed]
17. Weinstein A, Britchkov M. Lyme arthritis and post-Lyme disease syndrome. Curr Opin Rheumatol.2002;14:383–387. [PubMed]
18. Saulsbury FT. Lyme arthritis presenting as transient synovitis of the hip. Clin Pediatr (Phila)2008;47:833–835. [PubMed]
19. Thompson A, Mannix R, Bachur R. Acute pediatric monoarticular arthritis: distinguishing lyme arthritis from other etiologies. Pediatrics. 2009;123:959–965. [PubMed]
20. Margaretten ME, Kohlwes J, Moore D, et al. Does this adult patient have septic arthritis? JAMA.2007;297:1478–1488. [PubMed]
21. Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A:1629–1635. [PubMed]


