| Author | Affiliation |
|---|---|
| Jessica C. Schoen, BS, MS | University of California, Irvine School of Medicine, Irvine, CA |
| Megan M. Boysen, MD | University of California, Irvine, Department of Emergency Medicine, Irvine, CA |
| Chase R. Warren, BA | University of California, Irvine School of Medicine, Irvine, CA |
| Bharath Chakravarthy, MD, MPH | University of California, Irvine, Department of Emergency Medicine, Irvine, CA |
| Shahram Lotfipour, MD, MPH | University of California, Irvine, Department of Emergency Medicine, Irvine, CA |
ABSTRACT
The presentation of vertebrobasilar artery occlusion varies with the cause of occlusion and location of ischemia. This often results in delay in diagnosis. Areas of the brain supplied by the posterior circulation are difficult to visualize and usually require angiography or magnetic resonance imaging. Intravenous thrombolysis and local-intra arterial thrombolysis are the most common treatment approaches used. Recanalization of the occluded vessel significantly improves morbidity and mortality. Here we present a review of the literature and a case of a patient with altered mental status caused by vertebrobasilar artery occlusion.
INTRODUCTION
Altered mental status is a common presenting complaint in the emergency department (ED). Cerebrovascular accidents (CVAs) are one of the most serious etiologies for altered mental status. Ischemic stroke accounts for 87% of CVAs.1 Of these, approximately 20% are the result of vertebrobasilar artery occlusion (VBAO).2–5 The mortality of VBAO can reach 80–95% without successful treatment.3,6–8 Making the diagnosis can be difficult as patients present with a variety of nonspecific signs and symptoms. Furthermore, the posterior circulation is difficult to visualize, often requiring the use of computed tomography angiography (CTA), magnetic resonance imaging (MRI) or magnetic resonance angiography (MRA).2–3 We present here a review of the literature and a case of a young patient with VBAO.
HISTORY
A 36-year-old Caucasian female presented to the ED with altered mental status. According to her family, the patient began complaining of headache and a stiff neck four days prior to admission. The headache was frontal and not relieved with ibuprofen. She had no history of trauma. Approximately 13 hours prior to admission, the patient was evaluated at an outside ED and found to have an elevated white blood cell (WBC) count. A head CT was recommended for her headache, but she refused further workup. A presumptive diagnosis of sinusitis was made and she was treated with amoxicillin and ciprofloxacin/dexamethasone otic suspension and discharged home.
After discharge, her symptoms failed to resolve. According to her family, she complained of blurred vision and was noted to have unsteady gait and slurred speech. Prior to her call to emergency medical services (EMS), she called her family complaining that she felt “drunk.” Upon EMS arrival, she was unconscious.
Her past medical history was significant only for Turner’s syndrome. Her surgical history included breast reconstruction at age 15 years. She did not use ethanol, tobacco or illicit drugs. Home medications included amoxicillin, ciprofloxacin/dexamethasone, ibuprofen and pseudoephedrine.
PHYSICAL EXAM
Upon arrival to the ED, the patient was unresponsive. She was intubated and sedated. Blood pressure was 86/46 mmHg, heart rate 179 beats per minute, temperature 36.7°C and oxygen saturation 100% on oxygen after intubation. The pupils were 4mm, round and reactive to light; the oropharynx was clear; the neck was supple. The heart was regular and tachycardic and the lungs were clear bilaterally. The abdomen was soft, nontender and nondistended with active bowel sounds. The extremities showed no cyanosis, clubbing, nor edema, and peripheral pulses were palpated bilaterally. The skin showed no evidence of rash or lesions. The eyes were open and she was nonverbal with decerebrate posturing; the Glascow Coma Scale (GCS) score was seven. The gag reflex and corneal reflexes were grossly intact; doll’s eye sign was negative. The deep tendon reflexes were hyperreflexic throughout.
DIAGNOSTIC TESTING
A complete blood count included a WBC count of 10,500 cells/mcL with 85% neutrophils, hemoglobin 14.5 g/dL and hematocrit 42.6%. Electrolytes were: sodium 141 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L and carbon dioxide 21 mEq/L. Venous blood gas showed pH 7.41, pCO232.1, pO2 40.5 and bicarbonate 20.1 mEq/L. Renal and liver function tests, cardiac enzymes, coagulation cascade and urinalysis were normal. Blood alcohol and urine pregnancy tests were negative. Urine toxicology was positive for benzodiazepines, presumably from medications administered for sedation. A lumbar puncture was performed and cerebrospinal fluid (CSF) showed 179 red blood cells (RBCs) (tube #1), 56 RBCs (tube #4), 0 WBCs (tube #1), glucose 107 mg/dL and protein 32 mg/dL. The CSF was negative for West Nile Virus, Herpes Simplex Virus and Cryptococcus; no bacteria, viruses or fungi were isolated in culture.
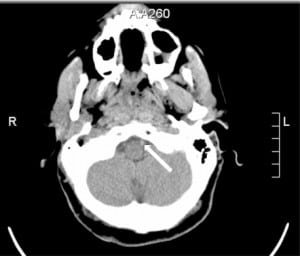
The initial electrocardiogram (ECG) showed sinus tachycardia at 179 beats per minute and premature ventricular contractions. Following intubation, ECG showed sinus tachycardia at 124 beats per minute. A single view chest radiograph was negative. A head CT without contrast was initially read as negative by the overnight teleradiologist. An in-house resident’s subsequent reading was positive for a high-density lesion in the left vertebral and basilar arteries, suspicious for thrombus versus slow flow (Figure 1). Incidentally, the CT also showed sinus mucosal disease. Follow-up CTA of the head and neck showed lack of opacification of the majority of the basilar artery and distal left vertebral artery.
The patient was taken immediately to the interventional radiology suite for vertebrobasilar artery (VBA) thrombectomy. Angiography of the VBA system showed dissection of the left vertebral artery with extensive thrombi in the left vertebral and basilar arteries. The thrombi were evacuated. Unfortunately, there was extravasation of contrast dye from the left posterior cerebral artery into the subarachnoid space (Figure 2). Post-procedure head CT showed evidence of reperfusion hemorrhage (Figure 3). After the procedure, the patient had further neurological decline and was transferred to the medical intensive care unit where she later expired. Autopsy confirmed VBAO as the cause of death. The patient had no abnormalities of the heart, including a normal foramen ovale.
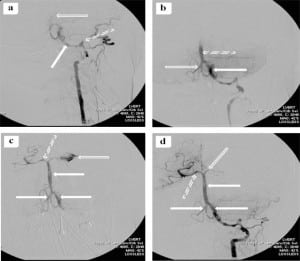
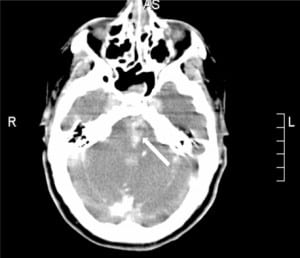
DISCUSSION
Clinical Presentation and Etiology
The clinical presentation of VBAO varies with the area of ischemia and cause of occlusion. Vertigo, dizziness, nausea, vomiting and head or neck pain are the most common initial symptoms reported.3,9–12 Other common signs and symptoms include weakness, hemiparesis, ataxia, diplopia, pupillary abnormalities, speech difficulties and altered mental status.2–3,10,12
Vertebral artery occlusion results in proximal VBA territory ischemia.11,13 Occlusion near the origin of the vertebral artery (extracranial) causes ischemia in the medulla and/or cerebellum and commonly presents as brief transient ischemic attacks (TIAs).3,14 Occlusion of an intracranial vertebral artery can cause ischemia in the lateral medulla resulting in Wallenburg Syndrome (decreased pain/temperature of the ipsilateral face and contralateral body, Horner’s syndrome, limb ataxia, hoarse voice, dysphagia).3 Dizziness, diplopia and signs of lateral medullary or cerebellar ischemia may result from extension of or embolism from an extracranial vertebral artery dissection (VAD) into the intracranial vertebral artery.3,14 Ischemia in the middle VBA territory is usually caused by occlusion of the basilar artery. This can cause pontine damage resulting in “Locked-In Syndrome” (quadriplegia, anarthria, preserved consciousness).13,15 Distal basilar artery occlusion is most commonly embolic, usually from cardiac or vertebral artery sources, and results in ischemia of the rostral midbrain and thalamus.11,13 This presents with the characteristic “top-of-the-basilar syndrome” (coma, midbrain ocular-motor signs – small poorly reactive pupils and defective vertical gaze, hemiparesis, hemiataxia) (Figure 4).2–3,9
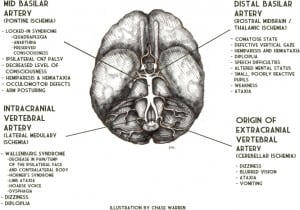
Embolism (30–40%), local atherothrombosis (15–35%) and VAD (approximately 5%) are the primary causes of VBAO.3,13,16–17 In elderly patients, local atherothrombosis is the most common etiology.9,11 These patients typically have multiple vascular risk factors and slow, progressive symptoms. TIAs weeks or months before the occlusion are common.2–3,9–11 VBAO in younger patients is most commonly caused by embolism, usually from cardiac, aortic, or proximal vertebral and basilar artery sources.2–3,9,11,13,16 These patients generally have few vascular risk factors and sudden symptom onset without prodromal TIAs.9,11
As in this case presentation, VAD occurs in the freely movable first and third segments of extracranial vertebral arteries and is commonly associated with minor trauma, such as coughing, sneezing, or vomiting, cervical chiropractic manipulation and whiplash injury.18–22 However, there is little evidence that minor trauma is a true risk factor for VAD in healthy vessels. Many occur spontaneously, suggesting an underlying arteriopathy or multifactorial etiology.18–19,21–23 Ehlers-Danlos syndrome, hyperhomocystinemia, recent infection and α1-antitrypsin deficiency have all been implicated.18,21–22 Turner Syndrome (TS) may have been an additional risk factor in the case presented here. Aortic root dilation and dissection are well known cardiovascular complications of TS.24–25 Some evidence suggests this may be related to an underlying “connective tissue disorder” characterized by cystic medial necrosis, similar to that seen in Marfan’s Syndrome.24–25 Ostberg et al26 demonstrated widespread structural vascular differences in women with TS compared to normal controls. To our knowledge, the case presented herein is the second reported case of spontaneous VAD in a patient with TS.27 VBAO results from thromboembolism or propagation of the dissection into the intracranial vertebral artery.3,10
Diagnosis
The initial workup of VBAO includes a non-contrast head CT to rule out intracranial hemorrhage.2,9,28 CTA is the most common subsequent study. MRI and MRA are alternative studies.28–30 Transcranial Doppler ultrasound may identify a high-resistance flow pattern or sudden signal loss as indicators of VBAO, but this technique depends on a skilled operator and is vastly inferior to CTA.2,9,29,31 While intra-arterial digital subtraction angiography is considered the criterion reference, it is invasive, time consuming, has limited availability, and may require general anesthesia.2
Recommended ancillary studies include echocardiography and rhythm monitoring to detect a cardiac source of embolism. Younger patients, those with prior occlusions, or those with no identifiable cardiac, aortic, or cervicocranial lesions, merit further workup for coagulopathy.3
Morbidity and Mortality
Recanalization of the occluded vessel is the most important prognostic factor in patients with VBAO.6,8,32–35 With successful recanalization, mortality is reduced by approximately 50%, survival is as high as 60% at three months and eventual functional independence is achieved in greater than 50% of patients.6,33–36
Other factors associated with good prognosis include younger age (<60 years), involvement of a single posterior circulation territory, and less severe presentation on admission (GCS>10, lower NIH Stroke Scale score, and absence of coma).6,11,13,16,32–33,37–38 Reports on the relationship between site of occlusion (distal vs. proximal) and prognosis are conflicting.6,11,13,16,33,35–36,39It is also debated whether shorter time from symptom onset to intervention improves morbidity and mortality. Most agree, however, that diagnosis delayed by as much as 48 hours should not preclude treatment.7–8,17,35–37
Our patient, although young, had a GCS of seven and involvement of two VBA territories, indicating a poor prognosis. Recanalization was achieved, but the patient suffered complications of thrombus evacuation, further worsening her prognosis. Treatment was initiated within 48 hours despite some delay in diagnosis.
Treatment
Available treatments include intravenous thrombolysis (IVT), local intra-arterial thrombolysis (IAT), endovascular thrombectomy and ultrasonic fibrinolysis.40
IVT and IAT are the most widely used strategies. IVT achieves recanalization in 43–67% of patients.8,17,34 It is noninvasive, can be administered earlier, and is more widely available than endovascular techniques.17,34 IAT may achieve recanalization in a higher proportion of cases (56–72%) than IVT, but does not necessarily improve patient outcome.7,17,34–35 Consequently, many authors recommend IAT as first-line treatment but encourage providers to treat with IVT if IAT is unavailable or will result in significant delay.7–8,17,34
Transcranial Doppler ultrasound may enhance IVT, and microcatheters with built-in ultrasonic devices have been used in conjunction with IAT.41–43 These studies demonstrate a trend toward higher rates of recanalization compared to controls treated with thrombolytic therapy alone. In the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, mechanical embolectomy achieved recanalization at rates comparable to those reported for IVT (46%) with a comparable risk of complication (13%).44
The safety and efficacy of these “thrombolytic adjuvants” for use in posterior circulation stroke are under investigation. Currently, most authors recommend reserving these treatments for patients ineligible for thrombolytic therapy.40
CONCLUSION
Altered mental status is a common presenting complaint in the ED. Ischemic stroke is an important differential diagnosis to consider even in younger patients. Arterial dissection must be considered as a potential etiology, particularly in patients with a known diagnosis of Turner Syndrome. The underlying connective tissue abnormality associated with Turner Syndrome may place these patients at much higher risk for spontaneous arterial dissection compared to the general population. VBAO, although a rarer type of ischemic stroke, can be devastating with mortality as high as 80–95% without successful treatment; early diagnosis therefore is crucial. Adequate visualization of the posterior circulation often requires angiography or magnetic resonance imaging. Intravenous thrombolysis and local-intra arterial thrombolysis are the most common treatment approaches used. Recanalization of the occluded vessel significantly improves the morbidity and mortality of VBAO.
Footnotes
We greatly acknowledge Dr. Shuichi Suzuki, Director, Division of Neruointerventional Radiology from UC Irvine Medical Center, for his contributions to the manuscript and for reviewing the images. We would also like to thank June Casey for her editorial assistance.
Supervising Section Editor: Rick A. McPheeters, DO
Submission history: Submitted July 30, 2010; Revision received October 21, 2010; Accepted October 29, 2010
Reprints available through open access at http://escholarship.org/uc/uciem_westjem.
Address for Correspondence: Shahram Lotfipour, MD, MPH, UC Irvine Medical Center, Department of Emergency Medicine, 101, The City Drive Rte 128, Orange, CA 92868
Email: shl@uci.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. American Heart Association Heart Disease and Stroke Statistics Writing Group Heart disease and stroke statistics – 2010 update: A report from the American Heart Association. Circulation.2010;121:e46–e215. [PubMed]
2. Baird TA, Muir KW, Bone I. Basilar artery occlusion. Neurocritical care. 2004;3:319–30.[PubMed]
3. Savitz SI, Caplan LR. Vetebrobasilar disease. N Engl J Med. 2005;352:2618–26. [PubMed]
4. Bogousslavsky J, Van Melle G, Regli F. The Lausanne stroke registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–92. [PubMed]
5. Vemmos K, Takis C, Georgilis K, et al. The Athens stroke registry: results of a five-year hospital-based study. Cerebrovasc Dis. 2000;10:133–42. [PubMed]
6. Brandt T, von Kummer R, Muller-Kuppers M, et al. Thrombolytic therapy of acute basilar artery occlusion: variables affecting recanalization and outcome. Stroke. 1996;27:875–81. [PubMed]
7. Lindsberg PJ, Soinne L, Roine RO, et al. Options for recanalization therapy in basilar artery occlusion. Stroke. 2005;36:203–4. [PubMed]
8. Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke. 2006;37:922–8. [PubMed]
9. Brandt T. Diagnosis and thrombolytic therapy of acute basilar artery occlusion: a review. Clinical and Experimental Hypertension. 2002;24:611–22. [PubMed]
10. Ferbert A, Bruckmann H, Drummen R. Clinical features of proven basilar artery occlusion.Stroke. 1990;21:1135–42. [PubMed]
11. Voetsch B, DeWitt LD, Pessin MS, et al. Basilar artery occlusive disease in the new England medical center posterior circulation registry. Arch Neurol. 2004;61:496–504. [PubMed]
12. von Campe G, Regli F, Bogousslavsky J. Heralding manifestations of basilar artery occlusion with lethal or severe stroke. J Neruol Neurosurge Psychiatry. 2003;74:1621–6.
13. Caplan LR, Chung C-S, Wityk RJ, et al. New England medical center posterior circulation stroke registry: I. Methods, data base, distribution of brain lesions, stroke mechanisms, and outcomes. J Clin Neruol. 2005;1:14–30.
14. Wityk R, Chang H, Rosengart A, et al. Proximal extracranial vertebral artery disease in the New England medical center posterior circulation registry. Arch Neruol. 1998;55:470–8.
15. Virgile RS. Locked-in syndrome: case and literature review. Clin Neurol Neurosurg.1984;86:275–9. [PubMed]
16. Caplan LR, Wityk R, Glass T, et al. New England medical center posterior circulation registry. Ann Neurol. 2004;56:389–98. [PubMed]
17. Schoenwille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. 2009;8:724–30. [PubMed]
18. Haneline M, Lewkovich G. An analysis of the etiology of cervical artery dissections: 1994–2003.J Manipulative Physiol Ther. 2005;28:617–22. [PubMed]
19. Haneline M, Triano J. Cervical artery dissection. A comparison of highly dynamic mechanisms: manipulation versus motor vehicle collision. J Manipulative Physiol Ther. 2005;28:57–63.[PubMed]
20. Dziewas R, Konrad C, Drager B, et al. Cervical artery dissection – clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179–84. [PubMed]
21. Rubinstein S, Peerdeman S, van Tulder M, et al. A systematic review of the risk factors for cervical artery dissection. Stroke. 2005;36:1575–80. [PubMed]
22. Schievnik WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med.2001;344:898–906. [PubMed]
23. Brandt T, Hausser I, Orberk E, et al. Ultrastructural connective tissue abnormalities in patients with spontaneous cervicocerebral artery dissections. Ann Neurol. 1998;44:281–5. [PubMed]
24. Bondy CA. Aortic dissection in Turner Syndrome. Curr Opin Cardiol. 2008;23:519–26.[PMC free article] [PubMed]
25. Elsheikh M, Dunger DB, Conway GS, et al. Turner’s Syndrome in adulthood. Endocr Rev.2002;23:120–40. [PubMed]
26. Ostberg JE, Donald AE, Halcox J, et al. Vasculopathy in Turner Syndrome: arterial dilation and intimal thickening without endothelial dysfunction. J Clin Endocrinol Metab. 2005;90:5161–6.[PubMed]
27. Muscat P, Lidov M, Nahar T, et al. Vertebral artery dissection in Turner’s Syndrome: diagnosis by magnetic resonance imaging. J Neruoimaging. 2001;11:50–3.
28. Davis SM, Tress BM, Bowling R, et al. Magnetic resonance imaging in posterior circulation infarction: impact on diagnosis and management. Aust NZ J Med. 1989;19:219–25.
29. Brandt T, Knauth M, Wildermuth S, et al. CT antiography and Doppler sonography for emergency assessment in acute basilar artery ischemia. Stroke. 1999;30:606–12. [PubMed]
30. Wentz KU, Rother J, Schwartz A, et al. Intracranial vertebrobasilar system: MR angiography.Radiology. 1994;190:105–10. [PubMed]
31. Ringelstein EB, Zeumer H, Poeck K. Non-invasive diagnosis of intracranial lesions in the vertebrobasilar system. A comparison of doppler sonographic and angiographic findings. Stroke.1985;16:848–55. [PubMed]
32. Gonner F, Remonda L, Mattle H, et al. Local intra-arterial thrombolysis in acute ischemic stroke.Stroke. 1998;29:1894–900. [PubMed]
33. Levy EI, Firlik AD, Wisniewski S, et al. Factors affecting survival rates for acute vertebrobasilar artery occlusions treated with intra-arterial thrombolytic therapy: a meta-analytical approach.Neurosurgery. 1999;45:539–45. [PubMed]
34. Lindsberg PJ, Soinne L, Tatlisumak T, et al. Long-term outcome after intravenous thrombolysis of basilar artery occlusion. JAMA. 2004;292:1862–6. [PubMed]
35. Sliwka U, Mull M, Stelzer A, et al. Long-term follow-up of patients after intraarterial thrombolytic therapy of acute vetebrobasilar artery occlusion. Cerebrovasc Dis. 2001;12:214–9. [PubMed]
36. Cross DT, III, Moran CJ, Akins PT, et al. Relationship between clot location and outcome after basilar artery thrombolysis. AJNR. 1997;18:1221–8. [PubMed]
37. Ezaki Y, Tustsumi K, Onizuka M, et al. Retrospective analysis of neurological outcome after intra-arterial thrombolysis in basilar artery occlusion. Surg Neurol. 2003;60:423–30. [PubMed]
38. Schonewille WJ, Algra A, Serena J, et al. Outcome in patients with basilar artery occlusion treated conventionally. J Neurol Neurosurg Psychiatry. 2005;76:1238–41. [PMC free article][PubMed]
39. Glass T, Hennessy P, Pazdera L, et al. Outcome at 30 days in the New England center posterior circulation registry. Arch Neurol. 2002;59:369–76. [PubMed]
40. Janjua N, Brisman JL. Endovascular treatment of acute ischemic stroke. Lancet Neurol.2007;6:1086–93. [PubMed]
41. Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–8. [PubMed]
42. Mahon BR, Nesbit GM, Barnwell SL, et al. North American clinical experience with the EKOS MicroLysUS infusion catheter for the treatment of embolic stroke. AJNR. 2003;24:534–8. [PubMed]
43. Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II Trial. AJNR. 2008;29:582–7. [PMC free article] [PubMed]
44. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–8. [PubMed]


