| Author | Affiliation |
|---|---|
| Charles R. Wira, III, MD | Yale School of Medicine, New Haven, CT |
| Emanuel Rivers, MD, MPH | Henry Ford Hospital, Detroit, MI |
| Brian Silver, MD | Henry Ford Hospital, Detroit, MI |
| Christopher Lewandowski, MD | Henry Ford Hospital, Detroit, MI |
ABSTRACT
Introduction:
In cerebral regions affected by ischemia, intrinsic vascular autoregulation is often lost. Blood flow delivery depends upon cardiac function and may be influenced by neuro-endocrine mediated myocardial suppression. Our objective is to evaluate the relation between ejection fraction (EF) and transcranial doppler (TCD) peak systolic velocities (PSV) in patients with cerebral ischemic events.
Methods:
We conducted a retrospective cohort study from an existing TCD registry. We evaluated patients admitted within 24 hours of onset of a focal neurological deficit who had an echocardiogram and TCD performed within 72 hours of admission.
Results:
We identified 58 patients from March to October 2003. Eighty-one percent (n=47) had a hospital discharge diagnosis of ischemic stroke and 18.9% (n=11) had a diagnosis of transient ischemic attack. Fourteen patients had systolic dysfunction (EF<50%). The mean PSV in patients with normal systolic function (EF≥50%) compared to those with systolic dysfunction (EF<50%) was as follows: middle cerebral artery 62.0 ± 28.6 cm/s vs. 51.0 ± 23.3 cm/s, p=0.11; anterior cerebral artery 52.1 ± 21.6 cm/s vs. 45.9 ± 22.7 cm/s, p=0.28; internal carotid artery 56.5 ± 20.1 cm/s vs. 46.4 ± 18.4 cm/s, p=0.04; ophthalmic artery 18.6 ± 7.2 cm/s vs. 15.3 ± 5.2 cm/s, p=0.11; vertebral artery 34.0 ± 13.9 cm/s vs. 31.6 ± 15.0 cm/s, p=0.44.
Conclusion:
Cerebral blood flow in the internal carotid artery territory appears to be higher in cerebral ischemia patients with preserved left ventricular contractility. Our study was unable to differentiate pre-existing cardiac dysfunction from neuro-endocrine mediated myocardial stunning. Future research is necessary to better understand heart-brain interactions in this setting and to further explore the underlying mechanisms and consequences of neuro-endocrine mediated cardiac dysfunction.
INTRODUCTION
Cerebral ischemic events (CIE) are a leading cause of death and morbidity in the United States with limited treatment options in the acute setting.1–6 This type of central nervous system injury has been associated with hemodynamic perturbation secondary to myocardial injury and dysfunction but the mechanisms underlying this relationship are poorly understood.5–7 Cardiac dysfunction in cerebral ischemia patients may have significant contributions in the pathogenesis of this disease. Within the territory of tissue affected by the ischemic insult, intrinsic autoregulation of the vasculature is lost rendering cerebral blood flow directly dependent upon cardiac output and contractility.8,9
The relationship between cerebral blood flow and cardiac contractility in the acute presentation of patients with acute cerebral ischemia has not been comprehensively evaluated. Our objective in this preliminary, retrospective study was to evaluate the relationship between left ventricular systolic function and transcranial doppler (TCD) peak systolic velocities (PSV) in patients with CIEs defined either as an acute ischemic stroke (AIS) or transient ischemic attack (TIA).
METHODS
The Institutional Review Board for human research approved the study protocol and procedures. We conducted a retrospective chart review of CIE patients identified by our institutions TCD registry.10We included patients based on the following criteria: adult patients admitted within 24 hours of onset of a focal neurological deficit, record of both an echocardiogram and TCD performed within 72 hours of admission, and a discharge diagnosis of an AIS or TIA. Our exclusion criteria included age less than 18 years old, the presence of cerebral hemorrhage on initial neuro-imaging, or defined imaging parameters on echocardiography which could confound results. Echocardiogram exclusion criteria included the inability to measure the cardiac ejection fraction (EF) or the presence of severe left-sided stenotic or regurgitant valvular lesions that could potentially alter cardiac output.
Additionally, given that certain TCD vessel distributions are technically more difficult to measure, we established a quality control measure to exclude individual vessel distributions from analysis if PSV or pulsatility index (PI) measurements could not be acquired in greater than 50% of patients. PSV vessel distributions included the middle cerebral artery, anterior cerebral artery, posterior cerebral artery, internal carotid artery, ophthalmic artery and vertebral artery. We measured PIs only in the middle cerebral and vertebral artery territories.
A senior resident investigator, under the supervision of senior faculty investigators, used a standardized method of data collection to extract demographic and clinical data onto a data collection form. Echocardiogram reports were read by staff cardiologists and reviewed for the measured EF and the presence of severe left-sided stenotic or regurgitant valvular lesions. We considered an EF below 50% abnormal.11
TCD studies in the registry were read by one of the investigators, a staff neurologist certified in neurosonology, and extracted by the senior resident investigator in a supervised and standardized fashion from the electronic medical record. We reviewed PSVs in the following vessel distributions: middle cerebral artery, anterior cerebral artery, posterior cerebral artery, internal carotid artery, ophthalmic artery and vertebral artery. We reported one PSV for each territory of the cerebral vasculature. We calculated the mean PSV for each vessel territory by combining the left and right PSV values in all patients. We performed the calculation this way because the PSV reference range for left and right vessel territories is identical and prior literature has demonstrated PSV and PI responsiveness in both sides of the middle cerebral artery territory to cardiac augmentation in the setting of AIS.12–14
Similarly, we measured the PI for each territory of the middle cerebral artery and vertebral artery, and calculated their means. We did not measure PIs in other vessel distributions.
Prior literature has documented PSV and PI to correlate with cerebral blood flow.14–20 For our primary analysis, we stratified patients into low (EF<50%) and normal (EF≥50%) contractility groups and calculated the mean PSV for each group. We performed a comparison of means using an unpaired t-test with statistical significance of the two-tailed P value being less than 0.05.
We reported the other results as the mean, standard deviation and 95% confidence interval where indicated. We used Fisher’s Exact Test in sub-group analysis comparing demographical differences between groups. We indicated statistical significance by an alpha error <0.05.
RESULTS
We identified 197 TCD studies during from March to October 2003. Of these studies, 127 were excluded for including neurosurgical patients (n=63), neurology patients with conditions other than CIEs (n=37), and other patient populations (n= 27). We identified 70 CIE patients with both TCD studies and echocardiograms performed during their hospitalization. However, we excluded 12 of these patients because the TCD and echocardiogram were not performed within the first 72 hours of admission, or because echocardiograms revealed severely stenotic or regurgitant left-sided valvular abnormalities. Thus, 58 patients fulfilled our inclusion and exclusion criteria and were included into the study cohort.
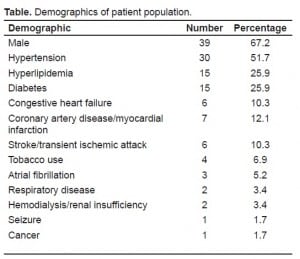
In the identified 58 patients, 81.1% (n=47) had a hospital discharge diagnosis of AIS and 18.9% (n=11) had a diagnosis of TIA. Demographics of our patient population are summarized in (Table). Male patients made up 67.2% of patients. The mean age was 65.0 ± 14.8. The most common co-morbidities were hypertension (51.7%), diabetes (25.9%) and hyperlipidemia (25.9%). Within the cohort, 10.3% of patients previously had an AIS or TIA and 10.3% of patients had a prior history of congestive heart failure. The mean initial NIH stroke scale for identified patients was 10.77 ± 7.29 (95% CI 7.54 to 14.00). The mean initial systolic blood pressure for patients was 168.7 mmHg ± 37.2 (95% CI 157.6 mmHg to 179.9 mmHg). The mean initial heart rate was 83.6 ± 16.7 (95% CI 78.4 to 88.7).
Of the total sample of patients, 24.1% (n=14) had evidence of systolic dysfunction with an EF below 50% on echocardiography, and the rest, 75.8% (n=44), had normal systolic function. Among patients with systolic dysfunction 14.3% (n=2) had a hospital discharge diagnosis of TIA. In the group with normal cardiac function 20.4% (n=9) had a hospital discharge diagnosis of TIA (p=1.0, Fisher’s Exact Test).
For TCD quality control, we ensured an adequate amount of PSV and PI measurements for each vessel distribution. We found that all vessel distributions met our quality threshold except for PSV measurements in the posterior cerebral artery distribution. We were only able to obtain 50.0% (n=29) of those measurements and therefore excluded the posterior cerebral artery vessel distribution from analysis. We performed PSV measurements for the middle cerebral artery distribution in 91.4% of patients (n=53), the anterior cerebral artery in 86.2% (n=50), the internal carotid in 88.0% (n=51), the ophthalmic artery in 69.0% (n=40), and the vertebral artery in 96.6% (n=56). For PI measurements, 91.4% (n=53) had middle cerebral artery PI measurements, and 96.6% (n=56) had vertebral artery PI measurements.
The mean PI in patients with normal systolic function (EF ≥50%) compared to those with systolic dysfunction (EF < 50%) was as follows: middle cerebral artery 1.07 ± 0.35 vs. 1.19 ± 0.41, p=0.23; vertebral artery 1.18 ± 0.34 vs 1.21 ± 0.28, p=0.62. We did not measure PIs in any other vessel distributions.
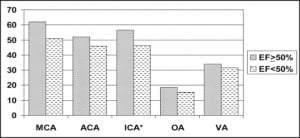
The mean PSV in patients with normal systolic function (EF ≥50%) compared to those with systolic dysfunction (EF < 50%) was as follows (Figure): middle cerebral artery 62.0 ± 28.6 cm/s vs. 51.0 ± 23.3 cm/s, p=0.11; anterior cerebral artery 52.1 ± 21.6 cm/s vs. 45.9 ± 22.7 cm/s, p=0.28; internal carotid artery 56.5 ± 20.1 cm/s vs. 46.4 ± 18.4 cm/s, p=0.04; ophthalmic artery 18.6 ± 7.2 cm/s vs. 15.3 ± 5.2 cm/s, p=0.11; vertebral artery 34.0 ± 13.9 cm/s vs. 31.6 ± 15.0 cm/s, p=0.44.
DISCUSSION
A significant proportion (13–28%) of patients with cerebral ischemia may have impaired cardiac contractility either at baseline from existing congestive heart failure or as part of the pathogenesis of the disease.21–23 The latter pathogenic mechanisms include catecholamine induced stunning or direct neuro-inhibition secondary to the ischemic insult.24,25 Our data suggests that cerebral blood flow (based on mean TCD PSV) in the internal carotid artery vascular territory is higher in acute cerebral ischemia patients with preserved left ventricular contractility. Additionally, we observed a trend towards higher cerebral blood flow among patients with preserved cardiac contractility in the territories of the middle cerebral artery, anterior cerebral artery, ophthalmic artery and vertebral artery. These findings serve to strengthen the limited existing animal literature and human pilot studies evaluating the impact of cardiac function on cerebral blood flow.
Nearly two decades ago Keller et al8 demonstrated in a primate model that cerebral blood flow in both ischemic and non-ischemic brain regions correlates with cardiac output. Soon afterwards, Korosue et al26 observed a 29% mean increase in cardiac output in ischemic stroke patients with isovolemic hemodilution associated with increases in regional cerebral blood flow.
More recently, Treib et al14 performed a pilot study with hypervolemic hemodilution combined with dopamine or dobutamine infusion in 24 stroke patients with middle cerebral artery occlusions. They measured the influence of blood pressure and cardiac output on the TCD PSV in a single intracranial vessel, the middle cerebral artery. With therapy there was a 53% increase in cardiac output. In the unaffected hemisphere, there was a 27% increase in the systolic velocity. On the side of the lesion, there was an 11% increase. The relative diminished response on the affected side was believed to have been due to thrombus burden or counterregulatory pathways causing vasoconstriction. Additionally, they observed an increase in PI by 46% in the affected and 47% in the unaffected hemisphere. This differed from our study since we did not observe significant PI differences between the low and normal EF groups. A significant difference between our studies is that Treib et al’s14patients received infusions of dopamine or dobutamine.
A translational question of important consideration is whether cardiac contractility correlates with functional neurological outcome. It is unknown if undifferentiated systolic dysfunction promotes expansion of the ischemic penumbra, or conversely whether hyperdynamic cardiac function enhances the risk of hemorrhagic transformation. Although relatively uninvestigated, Korosue et al26 reported modest increases in neurological scores with cardiac output augmentation. Subsequent isovolemic hemodilution studies were not supportive, but to our knowledge they did not record cardiac output values.27 No other studies have reported improved functional outcome with cardiac augmentation or hemodynamic optimization. Additionally, we found no studies relating higher cerebral hemorrhage rates to hemodynamic augmentation or hyperdynamic states.28,29
Nevertheless, in the stroke rehabilitation literature, Kevorkian et al30 observed better functional recovery in acute stroke patients with normal cardiac contractility. Their design differed from ours as they stratified acute stroke patients into severely depressed (EF<35%) and moderately depressed or normal (EF≥35%) contractility cohorts. The latter group had higher hospital discharge functional scores and higher rates of functional status improvement from admission to discharge. While the authors conceded their study had several limitations, their results suggest that acute stroke patients with preserved systolic function may have better functional outcomes than those with severe systolic dysfunction.
Thus, it remains unclear which subgroups of cerebral ischemia patients could potentially benefit from cardiac output augmentation as suggested by prior authors.31 Beyond impaired contractility, it is well-known that subgroups of cerebral ischemia patients may have serum troponin elevation and arrhythmias — which have been related to higher in-hospital mortality rates.21,24,32,33 Currently there is a paucity of investigators evaluating the incidence, role, underlying mechanisms, and impact of myocardial and hemodynamic derangements in the setting of cerebral ischemia. The future development of adjunctive evidence-based hemodynamic guidelines is critical.
LIMITATIONS
A principle limitation of this study was the retrospective design and small sample size. In addition, echocardiograms performed by technicians in our institution’s cardiovascular lab were susceptible to user variability. Another limitation was the heterogeneity of the defined CIE population. While there are neurovascular, physiologic and management differences between AIS and TIA patients, we postulated that the merging of these populations would not significantly impact our results for the sake of this preliminary analysis. For example, at our institution all AIS and TIA patients have echocardiograms and a cerebral vascular study performed prior to discharge. There are overlapping co-morbidities between each population, including diabetes, hypertension and hyperlipidemia. National AIS and TIA guidelines have overlapping recommendations for management, and at our institution AIS and TIA patients were admitted to the same clinical floors and clinician teams.34,35Thus, given the overlapping characteristics of stroke and TIA patients, we thought that the relation between cerebral flow velocities and cardiac function would be preserved.
Another limitation of our study was that we did not know the prior EF of patients to delineate the underlying mechanism for systolic dysfunction. It is possible that patients with elevated catecholamine levels from neuro-endocrine activation could potentially have myocardial stunning and other cerebrovascular perturbations that could alter echocardiogram findings and other variables measured in this study.
A methodological limitation of the retrospective design was that TCDs and echocardiograms were performed within 72 hours of admission, rather than simultaneously. Our study was also susceptible to selection bias since many patients undergoing TCDs may have had contraindications to magnetic resonance imaging or magnetic resonance angiography. Another limitation was that the majority of patients in our study did not have concomitant cerebral angiography performed in order to localize vascular occlusions in the AIS patients. Thus, our mean PSV or PI values could have been impacted if a patient had a partial or total occlusion of one of the large vessels being measured by TCD. For example, a patient could have a middle cerebral artery occlusion contrasted to a stroke syndrome caused by a more distal occlusion in the M1 or M2 portion of the middle cerebral artery territory. While our methodology to calculate the mean PSV or PI would help to mitigate this issue, it is a limitation that we were unable to control for given the information in the medical records and the fact that the majority of these patients did not have cerebral angiography performed.
CONCLUSION
Cerebral blood flow in the internal carotid artery territory as measured by the mean PSV in cerebral ischemia patients appears to be higher in those with preserved left ventricular contractility. Future research is necessary to better understand the impact of myocardial contractility on cerebral blood flow in patients with acute cerebral ischemia.
Footnotes
Supervising Section Editor: Kurt R Denninghoff, MD
Submission history: Submitted: August 31, 2009; Revision received January 26, 2010; Accepted April 29, 2010
Reprints available through open access at http://escholarship.org/uc/uciem_westjem.
Address for Correspondence: Charles R. Wira III, MD, Department of Emergency Medicine, Yale Acute Stroke Service, Yale School of Medicine, 464 Congress Ave., New Haven, CT 06510
Email: charles.wira@yale.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Lewandowski C, Barsan W. Treatment of acute ischemic stroke. Annals of Emergency Medicine.2001;37:202–16. [PubMed]
2. Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000;283:1151–8. [PubMed]
3. Johnston KC, Mao Y. National stroke surveillance program needed in Canada. CMAJ.2001;165:886–7.
4. Heuschmann PU, Berger K, Misselwitz B, et al. Frequency of thrombolytic therapy in patients with acute ischemic stroke and the risk of in-hospital mortality: the German Stroke Registers Study Group.Stroke. 2003;34:1106–13. [PubMed]
5. Oppenheimer SM, Hachinski VC. The cardiac consequences of stroke. Neurol Clin. 1992;10:167–76. [PubMed]
6. Burch GE. A new electrocardiographic pattern observed in cerebrovascular accidents. Circulation.1954;9:719–23. [PubMed]
7. Norris JW, Hachinski VC, Myers MG, et al. Serum cardiac enzymes in stroke. Stroke. 1979;10:548–53. [PubMed]
8. Keller TS, McGillicuddy JE, LaBond VA, et al. Modification of focal cerebral ischemia by cardiac output augmentation. Journal of Surgical Research. 1985;39:420–32. [PubMed]
9. Keller TS, McGillicuddy JE, LaBond VA, et al. Volume expansion in focal cerebral ischemia: the effect of cardiac output on local cerebral blood flow. Clin Neurosurg. 1982;29:40–50. [PubMed]
10. Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27:305–8. [PubMed]
11. Pfisterer ME, Battler A, Zaret BL. Range of normal values for left and right ventricular ejection fraction at rest and during exercise assessed by radionuclide angiography. European Heart Journal.1985;6:647–55. [PubMed]
12. Babikian VL, Wechsler LR, editors. Transcranial Doppler Ultrasonography. Mosby-Yearbook, Inc; St. Louis, MO: 1993.
13. Liboni W, Allais G, Mana O, et al. Transcranial doppler for monitorring the cerebral blood flow dynamics: normal ranges in the italian female population. Panminerva Medica. 2006;48:187–91.[PubMed]
14. Treib J, Haass A, Koch D, et al. Transcranial doppler examination on effect of hemodynamics on cerebral autoregulation in acute cerebral infarct. Ultraschall Med. 1996;17:64–7. [PubMed]
15. White H, Venkatesh B. Applications of transcranial doppler in the ICU: A Review. Intensive Care Med. 2006;32:981–94. [PubMed]
16. Petty GW, Wiebers DO, Meissner I. Transcranial doppler ultrasonography: clinical applications in cerebrovascular disease. Mayo Clin Proc. 1990;65:1350–64. [PubMed]
17. Aaslid R, Markwalder TM, Nornes H. Non-invasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurgery. 1982;57:769–774.
18. Karadeniz U, Erdemli O, Ozatik MA, et al. Assessment of cerebral blood flow with transcranial doppler in right brachial artery perfusion patients. Ann Thorac Surg. 2005;79:139–46. [PubMed]
19. Rosengarten B, Kaps M. Peak systolic velocity doppler index reflects most appropriately the dynamic time course of intact cerebral autoregulation. Cerebrovasc Dis. 2002;13:230–234.[PubMed]
20. Clyde B, Resnick DK, Yonas H, et al. The relationship of blood velocity as measured by transcranial doppler ultrasonography to cerebral blood flow as determined by stable xenon computed tomographic studies after aneurysmal subarachnoid hemorrhage. Neurosurg.1996;38:896–905.
21. Wira C, Lewandowski C, Martinez-Capolino C, et al. Myocardial and hemodynamic abnormalities in patients with acute strokes and TIAs. Annals of Emergency Medicine. 2006;48:20.
22. Sorescu D, Turk RJ, Cain M, et al. Clinical and transthoracic echocardiographic predictors of abnormal transesophageal findings in patients with suspected cardiac source of embolism. Am J Med Sci. 2003;326:31–4. [PubMed]
23. Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke. 1996;27:691–4. [PubMed]
24. Barber M, Morton JJ, Macfarlane PW, et al. Elevated troponin levels are associated with sympathoadrenal activation in acute ischemic stroke. Cerebrovasc Dis. 2007;23:260–66. [PubMed]
25. Meyer S, Strittmatter M, Fischer C, et al. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004;15:357–61. [PubMed]
26. Korosue K, Ishida K, Hamano S, et al. Clinical, hemodynamic, and hemorheological effects of isovolemic hemodilution in acute cerebral infarction. Neurosurgery. 1988;23:148–53. [PubMed]
27. Scandinavian Stroke Study Group Multicenter trial of hemodilution in acute ischemic stroke.Stroke. 1988;19:464–71. [PubMed]
28. Mistri AK, Robinson TG, Potter JF. Pressor therapy in acute ischemic stroke. Stroke.2006;37:1565–71. [PubMed]
29. Jauch EC, Lindsell CJ, Adeoye O, et al. Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke. 2006;37:2061–5. [PubMed]
30. Kevorkian CG, Nambiar SV, Rintala DH. Low ejection fraction: effect on the rehabilitation progress and outcome of stroke patients. Am J Phys Med Rehabil. 2005;84:655–61. [PubMed]
31. Caplan L. Worsening in Ischemic Stroke Patients: Is it Time for a New Strategy? Stroke.2002;33:1443–45. [PubMed]
32. Barasch E, Kaushik V, Gupta R, et al. Elevated cardiac troponin levels do not predict adverse outcomes in hospitalized patients without clinical manifestations of acute coronary syndromes.Cardiology. 2000;93:1–6. [PubMed]
33. Roquer J, Rodríguez-Campello A, Gomis M, et al. Comparison of the impact of atrial fibrillation on the risk of early death after stroke in women versus men. J Neurol. 2006;253:1484–1489.[PMC free article] [PubMed]
34. Easton JD, et al. Definition and evaluation of transient ischemic attack. A scientific statement for healthcare professionals from the AHA/ASASC/CCC/CRIC/CCN/ICPVD. Stroke. 2009;40:2276–93.[PubMed]
35. Adams H, et al. Guidelines for the Early Management of Adults with Ischemic Stroke. A Guideline from the AHA/ASASC/CCC/CRIC. Stroke. 2007;38:1655–17. [PubMed]


