| Author | Affiliation |
|---|---|
| Steven R. Offerman, MD | Kaiser Permanente South Sacramento Medical Center, Department of Emergency Medicine, Sacramento, CA |
| Melissa Schaefer, MD | University of California Davis Medical Center, Department of Emergency Medicine, Sacramento, CA |
| Joseph G. Thundiyil, MD, MPH | Orlando Regional Medical Center, Department of Emergency Medicine |
| Matthew D. Cook | University of California San Diego Medical Center, Division of Medical Toxicology, Department of Emergency Medicine |
| James F. Holmes, MD, MPH | University of California Davis Medical Center, Department of Emergency Medicine, Sacramento, CA |
ABSTRACT
Introduction:
We sought to identify factors associated with need for mechanical ventilation (MV), length of intensive care unit (ICU) stay, length of hospital stay, and poor outcome in injection drug users (IDUs) with wound botulism (WB).
Methods:
This is a retrospective review of WB patients admitted between 1991–2005. IDUs were included if they had symptoms of WB and diagnostic confirmation. Primary outcome variables were the need for MV, length of ICU stay, length of hospital stay, hospital-related complications, and death.
Results:
Twenty-nine patients met inclusion criteria. Twenty-two (76%) admitted to heroin use only and seven (24%) admitted to heroin and methamphetamine use. Chief complaints on initial presentation included visual changes, 13 (45%); weakness, nine (31%); and difficulty swallowing, seven (24%). Skin wounds were documented in 22 (76%). Twenty-one (72%) patients underwent mechanical ventilation (MV). Antitoxin (AT) was administered to 26 (90%) patients but only two received antitoxin in the emergency department (ED). The time from ED presentation to AT administration was associated with increased length of ICU stay (Regression coefficient = 2.5; 95% CI 0.45, 4.5). The time from ED presentation to wound drainage was also associated with increased length of ICU stay (Regression coefficient = 13.7; 95% CI = 2.3, 25.2). There was no relationship between time to antibiotic administration and length of ICU stay.
Conclusion:
MV and prolonged ICU stays are common in patients identified with WB. Early AT administration and wound drainage are recommended as these measures may decrease ICU length of stay.
INTRODUCTION
Background
Six reported forms of botulism exist, including food borne, infantile, wound, iatrogenic, adult infectious (in vivo adult intestinal colonization), and inhalational. Infantile botulism is the most frequently reported form, followed by wound botulism. Although wound botulism remains a rare diagnosis, its incidence has been rising. 1,2,3 In California, where the majority of wound botulism cases have occurred, an average of 0.5 cases per year were reported from 1951–1987. However, from 1987–1998 there was a 20-fold increase to 9.9 cases per year.4 Early wound botulism cases were related to deep tissue infections in avascular body locations, until the mid-1980s when the first cases associated with injection drug use (IDU were reported. Since then, the vast majority of wound botulism cases are related to IDU.1,4 Although the reasons are not well understood, the majority of wound botulism cases originate in California. 1,2 To date, the largest reported series of wound botulism from injection drug use consists of 129 cases reported from United States public health records between 1951–1998. Remarkably, 114 (87%) of these cases were diagnosed in California. The extraordinarily high number of wound botulism cases reported in California was attributed to black tar heroin imported illicitly from Mexico, although a definite causal association could not be made.4,5
A previously published series of 20 patients with wound botulism suggested that early anti-toxin administration was associated with lower frequency and shorter duration of mechanical ventilation.6 Additional evidence from a study of 132 patients with food botulism suggests lower fatality and shorter disease course from early anti-toxin administration.1,7
Although wound botulism is a rare disease, it causes severe disability and potential for adverse outcomes. The treatment frequently entails prolonged hospitalization, consumption of scarce medical resources, and high healthcare costs. It is possible that early identification and intervention by emergency physicians (EPs) may influence hospital course of care and outcomes in these cases.
Goals of this Investigation
We sought to describe the characteristics of injection drug users (IDUs) with wound botulism and to identify factors associated with need for mechanical ventilation, length of intensive care unit (ICU) stay, length of hospital stay, hospital-related complications, and death. We hypothesized that early anti-toxin administration is associated with improved patient outcomes.
METHODS
We conducted a retrospective review examining parenteral drug abusers admitted to any of three University of California hospitals (UC Davis Medical Center, Sacramento, CA; UC San Francisco Medical Center, San Francisco, CA; and UC San Diego Medical Center, San Diego, CA) with a diagnosis of wound botulism between 1991–2005. This study was approved with exemption from formal review by each hospital’s respective institutional review board. Patients were identified by a search for hospital discharges with an ICD-9 code for “Botulism” (005.1) and a review of hospital pharmacy records for patients who received botulinum antitoxin during the study period. Patients were included who had a documented history of IDU and a confirmed diagnosis of wound botulism. Confirmation of wound botulism, required subjects to have: 1) Symptoms consistent with wound botulism (bulbar palsy and/or peripheral weakness), and 2) A confirmatory test, including serum detection of botulinum toxin by bioassay or polymerase chain reaction (PCR); electromyography (EMG) findings consistent with botulism; and/or isolation of C. botulinum from wound culture. Pediatric patients (<18 years) and those with incomplete medical records were excluded.
Study definitions were determined a priori. Three abstractors recorded data from medical records onto a standardized data collection instrument. Inter-rater reliability of the abstractors was not measured. At the time of data collection, abstractors were not blinded to outcome variables, however emergency department (ED) records were abstracted prior to review of hospitalization data. Data collected from the ED record included: patient’s chief complaint, initial vital signs, symptoms, history of illicit drug use, physical examination findings, initial laboratory values, initial chest radiograph findings, negative inspiratory force (NIF) measurement, and ED interventions (oxygen administration, mechanical ventilation, antibiotic administration, anti-toxin administration, wound incision and drainage). Historical data was abstracted from narrative sections of the medical record.
The presence and timing of the following variables were documented from the hospital admission records: time from ED presentation to antitoxin administration in hours, length of ICU stay in hours, length of hospital stay in hours, length of mechanical ventilation in hours, length of time to incision and drainage (I&D) in hours, time to antibiotic administration in hours, chest radiograph findings for pneumonia, and hospital-related complications. Patients transferred to an outside hospital or nursing facility were excluded from hospital length of stay analyses as it was not feasible to document the length of stay in these patients. Hospital-related complications were defined as: the development of hospital-acquired pneumonia; venous line infections; pneumothorax during mechanical ventilation; development of a decubitus ulcer; development of venous thromboembolic disease, including deep venous thrombosis and pulmonary embolus; C. difficile colitis; and medication allergy. Pneumonia was defined by chest radiograph finding of infiltrate (by radiology report) with a documented diagnosis of “pneumonia” in the clinical notes. Hospital-acquired pneumonia was defined as pneumonia that was discovered after a normal ED chest radiograph.
Primary outcome variables were the need for mechanical ventilation (either endotracheal intubation or noninvasive ventilation), length of ICU stay, length of hospital stay, hospital-related complications, and death.
Data analysis
The study population was described using simple descriptive statistics. Continuous variables are presented as the mean ± one standard deviation for normally distributed variables and as the median with inter-quartile ranges (IQR) for variables with non-normal distribution Ninety-five percent confidence intervals (CI) are presented where appropriate. Differences in outcomes for continuous variables are compared using the Mann-Whitney two-sample test. Relationships between continuous variables and the outcomes of interest were measured using single variable linear regression analysis. Data analysis was performed with Stata statistical software (Release 8.0. College Station, TX: Stata Corporation; 2004).
RESULTS
Characteristics of Study Subjects
Seventy-two patients with an ICD-9 diagnosis of “Botulism” or receiving botulism anti-toxin were identified. Forty-three cases were excluded because patients were diagnosed with other forms of botulism (15); alternative neuromuscular diseases (5); or medical records were unavailable (23). Twenty-nine (59%) patients met study inclusion criteria. Case distribution was as follows: UC Davis Medical Center, 17 (59%); UC San Francisco Medical Center, 10 (34%); and UC San Diego Medical Center, two (7%). The cohort consisted of 21 males (72%) and eight females with a mean age of 45.3 ± 7.9 years. A history of IDU was documented in all cases. IDU was reported as heroin by 22 patients (76%) and heroin and methamphetamine by seven (24%). The reported route of injection drug administration was solely subcutaneous (“skin popping”) in 16 (55%); solely intravenous in three (10%); and both intravenous and subcutaneous in 10 (34%). Data regarding other reported routes of drug use was not collected.
Wound botulism diagnosis was confirmed by bioassay in 14 (48%) patients; PCR in one (4%); EMG in 18 (62%); and wound culture in three (10%). Botulinum toxin type was documented in 16 (55%) cases. Toxin type was type A in 14 (88%) patients, type B in one patient, and “type A & B” in one patient. This last type likely refers to a botulinum confirmation test that did not differentiate type A from type B.
Main Results
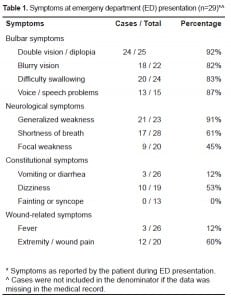
The most commonly recorded ED chief complaints were visual changes (13), including double vision (9) and blurry vision (4). Other chief complaints were weakness (9), difficulty swallowing (7), shortness of breath (4), speech changes (4) (including slurred speech [2] and difficulty speaking [2]), and dizziness (3). Sore throat, numbness, ptosis, arm & tongue heaviness, and altered mental status were reported in 1 patient each.
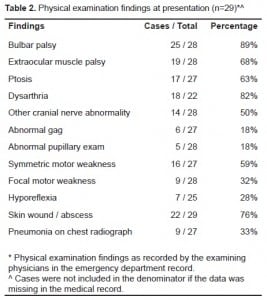
ED symptoms as reported by patients are presented in Table 1. The most common recorded symptoms were visual changes, speech changes, difficulty swallowing, and generalized weakness. All patients complaining of wound or extremity pain were found to have a soft tissue abscess. Five of the 22 patients with soft tissue wounds documented on physical examination did not have wound/extremity pain documented on presentation. ED physical examination findings are presented in Table 2. One patient did not have any documented ED physical examination. Vitals signs upon presentation were unremarkable, except for three patients with hypoxia by pulse oximetry, one with tachypnea, and one with bradypnea. No patient had a documented fever (>38°C) on presentation. Every patient in our sample received a complete blood count and chemistry panel in the ED. There were no laboratory abnormalities requiring therapeutic intervention. Data regarding blood cultures was not collected.
The median length of hospital stay was 628 hours (26.2 days) (IQR 192–1080 hours). Seven patients were discharged to an outside healthcare facility and one had no time of discharge recorded; therefore, complete data on hospital length of stay is not available. These eight patients were excluded from the hospital length of stay analysis only.
The median length of ICU stay was 348 hours (14.5 days) (IQR 51–716 hours). Twenty-one (72%, 95% CI 53–87%) patients were treated with mechanical ventilation. Length of ICU stay in patients who underwent mechanical ventilation was longer [636.6 hours (26.5 days); 95% CI 456.4–816.8 hours] than those without mechanical ventilation [36.4 hours (1.5 days); 95% CI 9.6–63.2 hours]. Noninvasive ventilation was attempted initially in three cases; however, all failed and ultimately underwent endotracheal intubation. Twenty hospital complications were documented in 13 patients. All complications occurred in mechanically ventilated patients (62% of intubated patients had a complication). Complications included: hospital-acquired pneumonia (10); C. difficile colitis (4); antitoxin allergy (2); pneumothorax (1); venous line infection (1; decubitus ulcer (1); venous thromboembolism (1). No patient (0%, 95% CI 0–10%) died.
Six patients (21%, 95% CI 8–40%) reported a previous presentation to a healthcare facility related to this illness. Those patients with a preceding presentation to a healthcare facility (missed diagnosis) did not have a higher rate of mechanical ventilation (4/6, 67%; 95% CI 22–97% versus 17/23, 74%; 95% CI 52–90%).
Botulinum antitoxin was administered to 26 (90%) patients. Each of these patients received a single vial of antitoxin. The median time to antitoxin administration was 15.3 hours (IQR 8–42 hours). Only two patients received antitoxin in the ED. The median time from hospital presentation to antitoxin administration was 16 hours (IQR 8–47 hours) in the MV group and 12 hours (IQR 7.5–29 hours) in the non-MV group (p=0.54). Linear regression analysis of the time from presentation to antitoxin administration versus length of ICU stay showed a highly significant relationship (Regression coefficient = 2.5; 95% CI 0.45–4.5; p=0.02). Two of the 26 patients (7.7%; 95% CI 0.9–25.1) receiving antitoxin had a documented allergic reaction. Both patients were intubated prior to antitoxin allergy. One was described as “mild” and the other “severe.” The mean time from presentation to antitoxin administration in the two patients who received antitoxin in the ED was 6.25 hours (95% CI = 3.8–8.7 hours) versus 44.65 hours (95% CI = 13.0–76.3 hours) in those patients not receiving antitoxin in the ED.
All 29 patients (100%) were treated with intravenous antibiotic medications during their hospitalization. The median time to antibiotics was 11 hours (IQR 4–22 hours). Linear regression analysis of time to antibiotics therapy versus ICU length of stay showed no significant relationship (Regression coefficient = −1.4; 95% CI −5.8–3.0; p=0.52)
Incision and drainage of a skin abscess was performed in 17 patients (59%). Of these, 15 had the time to wound incision and drainage documented. The median time to incision and drainage was 17 hours (IQR 9.5–34 hours). Linear regression analysis of time to wound incision and drainage versus length of ICU stay demonstrated a highly significant relationship (Regression coefficient = 13.7; 95% CI = 2.3–25.2; p=0.02).
DISCUSSION
These results demonstrate clinical characteristics of patients diagnosed with wound botulism related to IDU. All patients reported abusing heroin and the majority reported “skin popping” as the main route of administration. Chief complaints related to and physical findings of bulbar muscle palsy were documented in the majority of cases. In those cases without physical examination findings of bulbar palsy documented, the patient complaints suggested presence of a bulbar palsy in all cases. Nearly three-fourths of our cases had a skin wound identified, which is consistent with past series of wound botulism.4,6 Essentially all cases with documented serum toxin confirmation were found to have type A botulinum toxin, which is also consistent with prior reported series.1,4 Type A botulinum toxin is the most commonly reported serotype in the Western U.S.8
Patients with wound botulism had prolonged hospitalization and ICU stays. As expected, those who were mechanically ventilated had much longer stays than those not requiring mechanical ventilation. Additionally, all hospital-related complications occurred in the group of ventilated patients, with hospital-acquired pneumonia being the most common. In this study, we were unable to identify specific risk factors associated with patients requiring ventilation versus those not. This is unfortunate, as any strategy to prevent mechanical ventilation is likely to benefit the patient and significantly decrease resource utilization.
We found that the time interval from presentation to wound drainage correlated with ICU length of stay. Additionally, the time to wound drainage was shorter in those patients who did not require ventilation versus those requiring ventilation, but due to the limited sample size, these differences did not reach statistical significance. These associations indicate that early wound drainage may be important in decreasing ICU length of stay and resource utilization in patients with wound botulism.
The time from ED presentation to anti-toxin administration also correlated with ICU length of stay. Additionally, the time to anti-toxin administration was shorter in those patients who did not undergo mechanical ventilation versus those requiring ventilation, but again these differences did not reach statistical significance due to the small sample size. These associations indicate that early administration of anti-toxin may be important in decreasing ICU length of stay in these patients. Previous studies also suggest the importance of early anti-toxin administration in patients with wound botulism.1,6,7 Only two patients in our series received anti-toxin in the ED. While this finding is not surprising it is noteworthy, as the majority of patients in our study were seen at a facility that actually stocks botulinum anti-toxin in the hospital pharmacy, making ED administration more feasible. The delay in anti-toxin administration highlights the difficulty of diagnosing wound botulism and administering anti-toxin rapidly.
Wound Botulism poisoning is treated with equine-derived antitoxin. Bivalent anti-toxin (containing antibodies to type A and type B botulinum toxins) is the most commonly available formulation in the U.S.8 A trivalent antitoxin (type A, B, and E) is also available in certain locations. The antitoxin dosage is a single 10 mL vial diluted 1:10 in 0.9% saline administered by slow IV infusion. A second vial may be given in 2–4 hours if clinical progression is observed, but this is rarely necessary as the quantity of neutralizing antibodies within a vial of antitoxin usually far exceeds levels of circulating toxin. Antitoxin antibodies do not reverse paralysis but instead prevent further worsening of symptoms.3,8 For this reason, early antitoxin dosing is critical to stop symptom progression before the patient becomes severely affected and a prolonged hospital course is inevitable.
Unfortunately, the expense and difficulty of obtaining botulinum anti-toxin often discourages its early administration. A strategy of early, empiric dosing in selected, high-risk patients seems warranted and is likely to be overall cost-effective. Any IDU presenting to the ED with bulbar palsy should be considered for early treatment with botulinum anti-toxin.9 The finding of a skin wound should further increase suspicion and heighten the speed of anti-toxin administration. It is noteworthy that patients with botulism may initially present with asymmetric findings.10 Immediate testing of blood glucose, electrolytes, cranial CT scan, and lumbar puncture will help to rapidly narrow the differential diagnosis. Although there are many possible causes of symmetric motor neuropathies, both myasthenia gravis and the Miller-Fisher variant of Guillain-Barré syndrome may be confused with botulism, increasing the diagnostic difficulty.9,10 Furthermore, false positive edrophonium challenge tests (suggesting myasthenia gravis) have been reported in cases of wound botulism10 and food-borne botulism9,11,12 causing delays in diagnosis and treatment.
When the diagnosis of wound botulism is suspected, the process for anti-toxin acquisition should be immediately initiated. This requires contacting local (or state) health department authorities to facilitate anti-toxin delivery and specimen collection for confirmatory testing. Anti-toxin administration should generally not be delayed for botulism confirmation. Local health departments should contact the Centers for Disease Control (CDC) at (770) 488–7100 for assistance in acquiring anti-toxin. The CDC website (http://www.cdc.gov) contains detailed information about botulism epidemiology, diagnosis, confirmation, treatment and emergency response.
Although antitoxin administration should not be delayed for performance of electrophysiological testing, these studies may help to provide further diagnostic clarity while awaiting antitoxin acquisition. Characteristic electromyography (EMG) patterns have been described in association with botulinum toxin poisoning. Therefore, EMG testing may help to diagnose botulism or differentiate botulism from myasthenia gravis and Guillain-Barré syndrome.1,3,13 Expected findings with botulism are normal conduction velocities, small motor-action potentials, no change with repetitive stimulation, and fibrillation potentials with EMG.9,13 When electrophysiological testing is performed in the setting of suspected botulism, it is highly recommended that detailed descriptions of electrodiagnostic findings in botulism be reviewed prior to testing.3,14 EMG results are most reliable when performed by a person with experience in this procedure.
The criterion standard testing for botulism is the mouse bioassay procedure.1 Testing is usually performed for type A and type B botulinum toxins, as these are the most common toxin types within the U.S. Evaluation for type E toxin may be performed routinely in certain areas of the Pacific Northwest and Alaska or when a fish source of botulism is suspected.9 The mouse bioassay is performed by intra-peritoneal injection of mice with either pure subject serum or an incubated mixture of subject serum and monovalent anti-toxin. Monovalent antitoxins (Type A, B, C, D, E, F) are distributed by the CDC for testing purposes. Injected mice are then observed for four days for signs of botulism poisoning and death. It should be noted that although botulism usually kills mice within 6–24 hours, cases of delayed death are occasionally observed. If toxin is present all mice are expected to die, except those injected with the mixture containing the involved anti-toxin type.8,9An enzyme-linked immunosorbent assay (ELISA) test has also been developed, but is generally not available.9,15
LIMITATIONS
This study is limited by the restrictions inherent to the use of retrospective data, including incomplete documentation, recording bias, and changes in medical practice that may have occurred during the study period. Unfortunately, because wound botulism is a rare disease, prospective research in this area is difficult. Time estimates for ICU stay, antitoxin administration, and hospital stay may not be entirely accurate due to the retrospective nature of this study. Further, due to the social situations of this patient population, length of hospitalization may depend on factors other than medical necessity (e.g. placement, housing, rehabilitation, etc.). All study hospitals are academic centers where resident physicians are primarily responsible for initial patient evaluations and medical record documentation.
While it is likely that changes in intensive care practices did occur during the study period (1991–2005), there have been no significant changes in botulism anti-toxin formulation or known indications. Unfortunately, it was not feasible to adjust for changes in clinical practice over the study period when evaluating length of ICU stay and hospital complications. It is possible that in patients who were critically ill, drainage procedures and antitoxin administration may have been deferred in favor of more critical interventions (such as tracheal intubation), which could confound the observed associations between antitoxin, wound I&D, and ICU length of stay.
Due to the small sample, multivariate analysis to determine the independent contribution of multiple variables is not feasible. Wound botulism is a rare disease and therefore difficult to research. However, our study is the largest series of wound botulism cases in which hospital records were reviewed. Almost all botulism cases were type A; therefore, our results may not be generalizable to other botulinum toxin serotypes.
CONCLUSION
In this series of wound botulism cases, the time from ED presentation to antitoxin administration and wound drainage both correlated with ICU length of stay. Time of presentation to antibiotics was not associated with length of stay. In cases of suspected wound botulism, early antitoxin administration and wound drainage, possibly while the patient is in the ED, may help to decrease ICU length of stay.
Footnotes
Supervising Section Editor: Jeffrey R. Suchard, MD
Submission history: Submitted November 18, 2008; Revision Received April 1, 2009; Accepted April 15, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Steven R. Offerman, MD, Department of Emergency Medicine, Kaiser Permanente South Sacramento Medical Center, 6600 Bruceville Road, Sacramento, CA 95823
Email: steve.offerman@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Centers for Disease Control and Prevention: Botulism in the United States 1899–1996 Handbook for epidemiologists, clinicians, and laboratory workers. Atlanta, GA: Centers for Disease Control and Prevention; 1998.
2. Centers for Disease Control and Prevention Emergency Preparedness & Response Botulism: October62006. Available at: http://www.bt.cdc.gov/agent/botulism/ Accessed March 30, 2009.
3. Cherrington M. Botulism: update and review. Semin Neurol. 2004;24:155–63. [PubMed]
4. Werner SB, Passaro D, McGee J, et al. Wound botulism in California, 1951–1998: Recent epidemic in heroin injectors. CID. 2000;31:1018–24.
5. Passaro DJ, Werner SB, McGee J, et al. Wound botulism associated with black tar heroin among injecting drug users. JAMA. 1998;279:859–63. [PubMed]
6. Sandrock CE, Murin S. Clinical predictors of respiratory failure and long-term outcome in black tar heroin-associated wound botulism. Chest. 2001;120:562–6. [PubMed]
7. Tacket CO, Shandera WX, Mann JM, et al. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med. 1984;76:794–8. [PubMed]
8. Richardson WH, Frei SS, Williams SR. A case of type F botulism in Southern California. Clin Toxicol. 2004;42:383–7.
9. Horowitz BZ. Botulinum toxin. Crit Care Clin. 2005;21:825–839. [PubMed]
10. Horowtiz BZ, Swenson E, Marquardt K. Wound botulism associated with black tar heroin. JAMA.1998;280:1479–80.
11. Hughes JM, Blumenthal JR, Merson MH, et al. Clinical features of types A and B food-borne botulism. Ann Intern Med. 1981;95:442–5. [PubMed]
12. St Louis ME, Peck SH, Bowering D, et al. Botulism from chopped garlic: delayed recognition of a major outbreak. Ann Intern Med. 1988;108:363–8. [PubMed]
13. Merrison AF, Chidley KE, Dunnett J, et al. Lesson of the week: Wound botulism associated. BMJ.2002;325:1020–1. [PMC free article] [PubMed]
14. Cherington M. Electrophysiologic methods as an aid in diagnosis of botulism: a review. Muscle Nerve. 1982;5:S28–9. [PubMed]
15. Ferreira JL, Eliasberg SJ, Edmonds P, et al. Comparison of the mouse bioassay and enzyme-linked immunosorbent assay procedures for the detection of type A botulinal toxin in food. J Food Prot.2004;67:203–6. [PubMed]


