| Author | Affiliation |
|---|---|
| John Patka, PharmD | Grady Health System, Atlanta, GA |
| Daniel T. Wu, MD | Emory University, Atlanta, GA |
| Prasad Abraham, PharmD | Grady Health System, Atlanta, GA |
| Richard M. Sobel, MD, MPH | Southern Regional Hospital, Riverdale, GA |
ABSTRACT
Introduction:
To compare the effectiveness of ondansetron and prochlorperazine to treat vomiting. Secondary objectives were the effectiveness of ondansetron and prochlorperazine to treat nausea and their tolerability.
Methods:
This was a prospective, randomized, active controlled, double-blinded study. Using a convenience sample, patients were randomized to either intravenous ondansetron 4mg (n=32) or prochlorperazine 10mg (n=32). The primary outcome was the percentage of patients with vomiting at 0–30, 31–60, and 61–120 minutes after the administration of ondansetron or prochlorperazine. Secondary outcomes were nausea assessed by a visual analog scale (VAS) at baseline, 0–30, 31–60, and 61–120 minutes after the administration of ondansetron or prochlorperazine and the percentage of patients with adverse effects (sedation, headache, akathisia, dystonia) to either drug. We performed statistical analyses on the VAS scales at each time point and did a subgroup analysis to examine if nausea scores were affected if the patient had vomited at baseline.
Results:
The primary identified cause for nausea and vomiting was flu-like illness or gastroenteritis (19%). The number of patients experiencing breakthrough vomiting at 0–30, 31–60, and 61–120 minutes was similar between groups for these time periods; however, more patients receiving ondansetron experienced vomiting overall (7 [22%] vs. 2[3.2%] patients, p=not significant). Nausea scores at baseline and 0–30 minutes were severe and similar between groups; however, at 31–60 and 61–120 minutes, patients receiving prochlorperazine had better control of nausea (24.9 vs. 43.7 mm, p=0.03; 16.8 vs. 34.3 mm, p=0.05). Sedation scores were similar between groups. There were no cases of extrapyramidal symptoms as assessed by the treating physician and there were four cases of akathisia (prochlorperazine=3 [9%], ondansetron=1[3%]).
Conclusion:
Prochlorperazine and ondansetron appear to be equally effective at treating vomiting in the emergency department.
INTRODUCTION
Nausea and vomiting are common symptoms in the emergency department (ED). Antiemetic agents used to treat nausea and vomiting include phenothiazine derivatives, prokinetic agents and 5-HT3receptor antagonists. No agent is used uniformly for nausea and vomiting in the ED and recent studies include comparisons to ondansetron.1–8 This study compared prochlorperazine to ondansetron for the treatment of nausea and vomiting in the ED.
BACKGROUND
Chemical mediators involved in nausea and vomiting include dopamine, serotonin, histamine, norepinephrine and glutamine.9–11 These substances activate the chemoreceptor trigger zone (CTZ), found in the area postrema of the fourth ventricle. The CTZ then stimulates the vomiting center, which initiates the act of vomiting. The activation of 5-HT3 in the gastrointestinal (GI) tract can initiate vomiting by activating the vomiting center. Prochlorperazine and promethazine are phenothiazine antiemetics that have been used for the treatment of nausea and vomiting secondary to a wide range of pathologies. Their primary mechanism of action is antagonism of D2 receptors in the CTZ, although phenothiazines also have antihistaminic and anticholinergic properties. The 5-HT3antagonist ondansetron blocks serotonin in the CTZ and the gastrointestinal tract. Ondansetron has been examined in over 100 studies for the treatment of nausea and vomiting related to chemotherapy and anesthesia.10 There are limited data on the use of ondansetron in the ED, except for use in children.1,2
METHODS
This was a prospective, randomized, active controlled, double-blinded study designed to compare the effectiveness of ondansetron and prochlorperazine to treat vomiting. Secondary objectives included the effectiveness of ondansetron and prochlorperazine to treat nausea and the tolerability of prochlorperazine and ondansetron in patients with nausea and/or vomiting. Patients were eligible for inclusion if they were admitted to the ED with nausea and/or vomiting. Exclusion criteria included: previous treatment in the ED with antiemetics; missed last menstrual period or pregnancy; less than 18-years-old; conditions with impaired GI tract function (i.e. irritable bowel syndrome); impaired mental status; treatment with antineoplastic agents within seven days prior to randomization; patients unable to read, write, or communicate in the English language; patients leaving the ED against medical advice. Patients were assigned to treatment using a 1:1 random numbers table to 4mg of ondansetron intravenous (IV) push over 2–5 minutes, or 10 mg of prochlorperazine IV administered over two minutes.
Information collected on admission included: demographics, past medical history, social history, chief complaint, suspected cause of nausea and/or vomiting, number of episodes of vomiting prior to randomization, medications prior to admission and during study time period, duration of nausea and/or vomiting (per history), number of episodes of vomiting, and number of dry heave episodes. The presence or absence of extrapyramidal symptoms or akathisia was assessed by physician observation, and no scale or specific criteria were used to rate severity.
Study Outcomes
The primary outcome was the percentage of patients with vomiting at 0–30 minutes, 31–60 minutes and 61–120 minutes after the administration of ondansetron or prochlorperazine. Secondary outcomes included: nausea assessed by a VAS at baseline and three time intervals, 0–30 minutes, 31–60 minutes and 61–120 minutes, based on similar methods by Ernst et al5. We measured VAS scores from 0 to 100 mm with zero being no nausea and 100 mm the worst possible score. In addition, we stratified VAS scores into quartile ranges (none, mild, moderate and severe) to aid in the clinical application of the numerical data. Other outcomes included the percentage of patients experiencing adverse effects (sedation, headache, akathisia and dystonia) to ondansetron or prochlorperazine and treatment failures, defined as requiring rescue antiemetic treatment >30 minutes after administration of the study medication. We also assessed sedation and headache by a VAS with scores from 0 to 100 mm (none to most severe) and stratified them into quartile ranges (none, mild, moderate, severe). The need for a rescue treatment was based on physician preference. Rescue medications were not administered ≤30 minutes post administration of study medication. The decision to administer a rescue medication, as well as the medication choice, was up to the discretion of the treating physician and patients were not crossed over to the other treatment group. Blinding could be broken if there was a safety concern; however, this was not required in any patients.
RESULTS
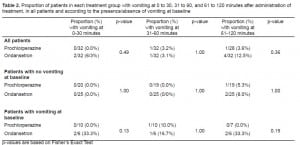
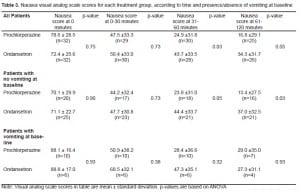
Three hundred fifty-three patients were screened and 64 patients were randomly assigned to either ondansetron or prochlorperazine. Reasons for exclusion consisted of: received prior treatment (38%), refused to participate (19%), history of impaired GI tract function (9%), altered mental status (7%), GI bleed (5%), unable to obtain IV access (3%), and unable to consent (19%). The primary cause for nausea and vomiting was flu-like illness or gastroenteritis (19%); other causes included hyperglycemia (6%), alcohol intoxication (5%), gastritis (5%), adhesions (3%), cholecystitis (3%), pancreatitis (2%), renal colic (2%), or undetermined (55%). Demographics for the study population are listed in Table 1. The number of patients experiencing breakthrough vomiting at 0–30 minutes, 31–60 minutes and 61–120 minutes is shown in Table 2. Results were similar between groups; however, overall more patients receiving ondansetron experienced breakthrough vomiting [7 (22%) vs. 2 (6.2%) patients, p=0.23]. In addition, one patient randomized to prochlorperazine, and five patients in the ondansetron group required rescue treatment during the study time period (p=0.20). One patient in the prochlorperazine group and four in the ondansetron group received 25mg promethazine IV, and one patient in the ondansetron group received metoclopramide 10mg IV. Results for nausea VAS scores are in Table 3; they were divided into four categories: none (0), mild (1–33), moderate (34–66) and severe (>66). Nausea scores at baseline and 0–30 minutes were severe and similar between groups, however at 31–60 and 61–120 minutes, patients receiving prochlorperazine had significantly lower nausea scores; the prochlorperazine group scores were mild and the ondansetron were moderate at 61–120 min. In a subgroup analysis, there was no difference in nausea scores if the patient had vomited at baseline. Data for the VAS was not complete for all subjects at all time points due to the inability to perform the VAS secondary to increased sedation (prochlorperazine 7 [22%], ondansetron 5 [16%]), or dropouts due to receiving rescue treatment (prochlorperazone 1 [3%], ondansetron 5 [16%]). Sedation scores were mild for both groups throughout the study period and not statistically different between groups, (p>0.05). Headache scores were mild in the prochlorperazine group at all time points, while moderate at baseline and 0–30 min and mild at 31–60 and 61–120 minutes with ondansetron. Headache scores were significantly lower in the prochlorperazine group at all times points, (p<0.05). There were no cases of extrapyramidal symptoms and four cases of akathisia (prochlorperazine=3 [9%], ondansetron=1[3%]).

DISCUSSION
These results demonstrate that prochlorperazine and ondansetron appear to be equally effective at controlling vomiting in patients presenting to the ED with nausea and/or vomiting. Theoretically prochlorperazine may have an advantage over ondansetron, as serotonin can cause the release of dopamine.12,13 If dopamine is already present, or released, during nausea or vomiting, blocking serotonin will not be able to prevent the action of this dopamine. Although the results were not statistically different, this may explain the improved response with prochlorperazine. Central 5-HT3activity may be more associated with nausea considering the benefit of ondansetron in the pre-treatment of nausea associated with chemotherapy and anesthesia, while receptors in the GI tract are important with treatment of emergent vomiting.
When examining the data on antiemetics in the ED, prochlorperazine and promethazine were studied in a randomized double-blind comparison of adults with gastritis or gastroenteritis. Patients were well-matched according to gender, age, duration and number of times vomiting, and baseline nausea as determined by a VAS. Eighty-four patients were randomized to IV prochlorperazine 10mg (n=42) or promethazine 25mg (n=42). Patients were then assessed at 30 minutes, 60 minutes and >60 minutes. The results showed that in the prochlorperazine group more patients demonstrated complete relief (determined by patients) within 30 minutes when compared to promethazine (33.4% vs. 16.7%, p=0.021). Results were 50 vs. 47.6% (30 to 60 min) and 16.7 vs. 35.7% (>60 minutes), with prochlorperazine and promethazine respectively. In addition, there were more treatment failures with promethazine compared to prochlorperazine, 13 vs. 4 patients (p=0.03). Two studies examined the effects of ondansetron compared to placebo in primarily pediatric patients admitted to the ED with acute gastroenteritis.1,2 In the first study (N=107) IV ondansetron resulted in complete cessation of vomiting more often compared to placebo (70% vs. 51%, p=0.04) during ED stay.1 Oral ondansetron was compared to placebo in 145 patients in the second study.2 During the ED stay, 87% of patients in the ondansetron group and 64% of patients in the placebo group experienced no emesis (p=0.004). Follow up at 24 and 48 hours showed no difference between groups in the number of episodes of emesis, or proportion of patients with emesis. Recently, ondansetron was compared to promethazine in the ED. One hundred twenty patients were randomly assigned to ondansetron 4mg or promethazine 25mg IV; those receiving prior antiemetics were excluded.8 Ondansetron and promethazine were found to be comparable, −34 mm vs. −36 mm, respectively, using a 100-mm VAS. In addition promethazine caused more sedation and there were two cases of akathisia in the promethazine group.
LIMITATIONS
Although this study had a strong design there were several limitations. Because we used a convenience sample with a small sample size the results of this study would need to be confirmed in a larger, powered study. We calculated a sample size of 300 patients would be needed to detect a 30% difference in vomiting between groups with a beta of 0.8 and an alpha of 0.05, estimating a 70% effectiveness rate with ondansetron. With a larger study a smaller, yet clinically important, difference in effectiveness may be determined. In addition, a higher percentage of patients in the ondansetron group (26 vs. 7%) were admitted to the hospital. These patients could potentially be considered at higher risk for nausea and vomiting due to a greater severity of illness and therefore explain the decreased response to ondansetron. All patients were not able to participate in post-treatment assessment VAS scores due to sedation, which reduced the sample size at each time interval. Headache scores were significantly lower in the prochlorperazine group; however, they were also significantly lower at baseline. The differences in scores at baseline could explain the lower headache scores overall in the prochlorperazine group. In this study 4mg of IV ondansetron was used, which is the dose typically used for nausea and vomiting at our institution. However, higher and lower doses of ondansetron have been shown to be effective.14,15 If a higher dose of ondansetron had been used there could have been improved control of nausea and vomiting with ondansetron compared to prochlorperazine. Finally, our rates of extrapyramidal symptoms or akathisia were low; however, physician self-reporting determined their presence. If a specific scale was used to detect extrapyramidal symptoms or akathisia their rates may have been higher than we reported.16
CONCLUSION
Prochlorperazine and ondansetron appear to be equally effective to treat vomiting in the ED. Prochlorperazine may be more effective in controlling nausea. Although this was a prospective, randomized, active controlled, double-blinded study, it had a small sample size and the results should be confirmed in a larger, powered study.
Footnotes
Supervising Section Editor: Gregory Moore, MD, JD
Submission history: Submitted November 5, 2009; Revision received February 3, 2010; Accepted April 18, 2010.
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: John Patka, PharmD, Department of Pharmacy and Drug Information, Grady Health System, 80 Jesse Hill Jr Dr., Atlanta, GA 30303
Email jpatka@gmh.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Reeves JJ, Shannon MW, Fleisher GR. Ondansetron decreases vomiting associated with acute gastroenteritis: a randomized, controlled trial. Pediatrics. 2002 Apr;109(4):e62. [PubMed]
2. Ramsook C, Sahagun-Carreon I, Kozinetz CA, et al. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med.2002 Apr;39(4):397–403. [PubMed]
3. Collins RW, Jones JB, Walthall JD, et al. Intravenous administration of prochlorperazine by 15-minute infusion versus 2-minute bolus does not affect the incidence of akathisia: a prospective, randomized, controlled trial. Ann Emerg Med. 2001 Nov;38(5):491–6. [PubMed]
4. Vinson DR, Drotts DL. Diphenhydramine for the prevention of akathisia induced by prochlorperazine: a randomized, controlled trial. Ann Emerg Med. 2001 Feb;37(2):125–31.[PubMed]
5. Ernst AA, Weiss SJ, Park S, et al. Prochlorperazine versus promethazine for uncomplicated nausea and vomiting in the emergency department: a randomized, double-blind clinical trial. Ann Emerg Med. 2000 Aug;36(2):89–94. [PubMed]
6. Drotts DL, Vinson DR. Prochlorperazine induces akathisia in emergency patients. Ann Emerg Med.1999 Oct;34(4 Pt 1):469–75. [PubMed]
7. Jones J, Pack S, Chun E. Intramuscular prochlorperazine versus metoclopramide as single-agent therapy for the treatment of acute migraine headache. Am J Emerg Med. 1996 May;14(3):262–4.[PubMed]
8. Braude D, Crandall C. Ondansetron versus promethazine to treat acute undifferentiated nausea in the emergency department: a randomized, double-blind, noninferiority study. Acad Emerg Med.2008;15(3):209–15. [PubMed]
9. Heilenbach T. Marx: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 5th ed. St. Louis, MO: Mosby, Inc; 2002. Nausea and Vomiting; pp. 178–185.
10. ASHP Therapeutic Guidelines on the Pharmacologic Management of Nausea and Vomiting in Adult and Pediatric Patients Receiving Chemotherapy or Radiation Therapy or Undergoing Surgery.Am J Health Syst Pharm. 1999 Apr;56(8):729–64. [PubMed]
11. Mitchelson F. Pharmacological agents affecting emesis. A review (Part I) Drugs. 1992 Mar;43(3):295–315. [PubMed]
12. Allan SG. Antiemetics. Gastroenterol Clin North Am. 1992;21(3):597–611. [PubMed]
13. Lindley C. Applied Therapeutics: The Clinical Use of Drugs. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. Nausea and Vomiting; pp. 8-1–8-18.
14. Bowhay AR, May HA, Rudnicka AR, et al. A randomized controlled trial of the antiemetic effect of three doses of ondansetron after strabismus surgery in children. Paediatr Anaesth. 2001 Mar;11(2):215–21. [PubMed]
15. Splinter WM, Rhine EJ. Low-dose ondansetron with dexamethasone more effectively decreases vomiting after strabismus surgery in children than does high-dose ondansetron. Anesthesiology.1998 Jan;88(1):72–5. [PubMed]
16. Drotts DL, Vinson DR. Prochlorperazine induces akathisia in emergency patients. Ann Emerg Med. 1999;34:469–75. [PubMed]


