| Author | Affiliation |
|---|---|
| Brian O’Neil, MD | Wayne State University, Department of Emergency Medicine, Detroit, Michigan |
| Leslie S. Prichep, PhD | New York University School of Medicine, Brain Research Laboratories, Department of Psychiatry, New York, New York |
| Roseanne Naunheim, MD | Washington University School of Medicine, Division of Emergency Medicine, St. Louis, Missouri |
| Robert Chabot, PhD | New York University School of Medicine, Brain Research Laboratories, Department of Psychiatry, New York, New York |
ABSTRACT
Introduction:
The incidence of emergency department (ED) visits for Traumatic Brain Injury (TBI) in the United States exceeds 1,000,000 cases/year with the vast majority classified as mild (mTBI). Using existing computed tomography (CT) decision rules for selecting patients to be referred for CT, such as the New Orleans Criteria (NOC), approximately 70% of those scanned are found to have a negative CT. This study investigates the use of quantified brain electrical activity to assess its possible role in the initial screening of ED mTBI patients as compared to NOC.
Methods:
We studied 119 patients who reported to the ED with mTBI and received a CT. Using a hand-held electroencephalogram (EEG) acquisition device, we collected data from frontal leads to determine the likelihood of a positive CT. The brain electrical activity was processed off-line to generate an index (TBI-Index, biomarker). This index was previously derived using an independent population, and the value found to be sensitive for significant brain dysfunction in TBI patients. We compared this performance of the TBI-Index to the NOC for accuracy in prediction of positive CT findings.
Results:
Both the brain electrical activity TBI-Index and the NOC had sensitivities, at 94.7% and 92.1% respectively. The specificity of the TBI-Index was more than twice that of NOC, 49.4% and 23.5% respectively. The positive predictive value, negative predictive value and the positive likelihood ratio were better with the TBI-Index. When either the TBI-Index or the NOC are positive (combining both indices) the sensitivity to detect a positive CT increases to 97%.
Conclusion:
The hand-held EEG device with a limited frontal montage is applicable to the ED environment and its performance was superior to that obtained using the New Orleans criteria. This study suggests a possible role for an index of brain function based on EEG to aid in the acute assessment of mTBI patients.
INTRODUCTION
Traumatic brain injury accounts for over 1 million emergency department (ED) visits annually within the United States with the majority of these visits for mild injury.1,2This incidence is increasing at an alarming rate, rising 21% from 2002 to 2006, quadrupling the rate of population growth. This increasing rate will further tax ED resources.
The American College of Emergency Physicians’ 2008 panel on mild traumatic brain injury (mTBI) raised several important issues, among them which patients with acute mTBI should have a non-contrast computed tomography (CT) in the ED. This question is particularly relevant given concerns over the increased use of CT and the long-term complications of radiation. The estimated increased cancer risk from a CT has been estimated to be 1 patient in 1000–2000. 3 In EDs the overwhelming majority of patients presenting with mTBI routinely undergo a CT. This occurs primarily because of the zero tolerance for missed intracranial lesions and because current decision rules for the use of CT in TBI have high sensitivity at the expense of poor specificity (that is, low false negative rate and a high false positive rate). 4–6
Quantitative electroencephalography (QEEG) has been shown to be a sensitive indicator of the presence of brain injury after mild head injury.7 QEEG can be used to distinguish normal controls from patients with mild head injury (mTBI),8,9 and patients with mild head injury from those with severe head injury.10 QEEG features appear to be sensitive for post-concussion syndrome and can predict recovery of function at one-year post injury11–13 and discriminant functions using derived features of brain electrical activity were demonstrated to be sensitive indicators of brain dysfunction after mild head injury due to blast concussion.14 Using such methods, classification of athletes with residual brain injury subsequent to concussion was also reported.15, Current evidence suggests that electrophysiological abnormalities reflecting functional changes in the brain may emerge earlier than structural changes and may better detect mTBI than conventional neuroimaging techniques. 16
Recent advances, including limited lead EEGs, improved automatic artifact detection, quantitative EEG analysis and the application of pattern recognition algorithms, have led to studies demonstrating the feasibility of using these technologies in the ED setting.17Further, recent publications in sports concussion using this approach have reported that an index derived from quantitative brain electrical activity (TBI Index) reflected significant persistence of brain dysfunction beyond the point of clinical recovery.18,19
The present study was designed to investigate whether the TBI-Index can play a role in the initial screening of mTBI patients presenting to the ED. More specifically, can it be shown to be useful in predicting which patients should be sent for further brain imaging studies such as CT for the determination of the presence of structural brain damage or which patients might be discharged without further testing? These results will be compared to those obtained using the New Orleans Criteria (NOC). To this end we used a hand-held device to collect EEG data in the ED environment. We processed this data off-line to obtain a single brain electrical activity measure (biomarker) in this independent population, using the index derived previously (unpublished data, see EEG Data Analysisbelow) in a separate mTBI ED population (n=282) and shown to be sensitive (>90%) for prediction of positive CT.
METHODS
Subjects
The study population consisted of a convenience sample of 119 ED patients who presented with acute head injury and received a CT. Patients were enrolled in the ED at 1 of the 8 study sites (the majority from Washington University, Barnes Hospital, Bellevue Hospital Center and Royal Oaks Medical Center), following a closed head injury (85% within 24 hours of injury) and meeting the inclusion/exclusion criteria described below. All sites received approval from their respective Human Research Committees. Written informed consent was obtained prior to testing of all subjects. For the purpose of this study, CTs were read as positive if they had lesions potentially due to trauma, including cerebral or cerebellar contusion, subarachnoid hemorrhage, parenchymal bleeds, petechial hemorrhages, subdural and epidural hematomas. We defined mTBI using the American Congress of Rehabilitation criteria, which requires that at least 1 of the following conditions be met: any period of loss of consciousness < 30 minutes; Glasgow Coma Scale (GCS) score of 13–15; any loss of memory for the event immediately before or after the injury, with post traumatic amnesia less than 24 hours; or any alteration in mental state at the time of the event, (dazed, disoriented or confused).
Inclusion/Exclusion Criteria
Eligible for study were patients over 18 years of age who presented to the ED after a closed head injury, met the above mTBI definition and had a CT ordered as part of their evaluation. Patient enrollment occurred during all periods when the research assistants were available; patients were not selected by referral from treating physicians. We excluded patients if clinical conditions would not allow placement of the electrodes or if they were unable (e.g., obtunded due to intoxication) or unwilling to provide informed consent. In addition, we excluded patients with chronic psychiatric disorder, chronic drug or alcohol abuse, or chronic seizure history. We also excluded developmentally delayed patients, or those who were taking central nervous system active medication that the investigator believed would interfere with the EEG testing. Finally, if the head injury was believed to be a result of a seizure, the patient was not a candidate for this study.
Design and Procedures
Evaluations were made in the ED by ED research assistants, none of whom had formal EEG experience. The evaluations were done as early as practical without hindering patient care. The mean time from injury to evaluation in the ED was <12 hours for the vast majority (∼80%) of the subjects and all were tested within 72 hours. All patients’ hospital records were queried after ED or hospital discharge. At the time of EEG evaluations the research assistants were also blinded to CT outcome and NOC score.
Computed Tomography
CT interpretations from final reports issued by the neuroradiologists at each institution as the final CT result for this study. The CT readings were made blinded to all other information about the patient, other than the TBI indication for the head scan. An independent investigator blinded to EEG and all other clinical results scored the CTs of the CT positive (CT+) group using the Marshall criteria.20 The Marshall criteria is a method for grading the severity of CT abnormality on a 6-point scale, where “I” indicates a diffuse injury with no visible pathology and “VI” indicates a non-evacuated mass lesion (>25cc).
New Orleans Criteria (NOC)
The queries that make up the NOC scores were collected by the research assistant at the time of the EEG evaluation, for scoring off site.4 These included: headache, vomiting, age > 60 years, drug or alcohol intoxication, persistent anterograde amnesia, visible trauma above the clavicle, or seizure.5 If the patient had any 1 of these items the NOC was considered to be positive.
EEG Acquisition
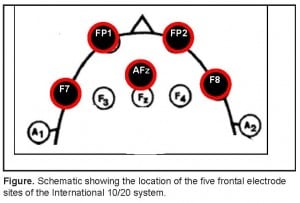
Patients underwent 10 minutes of eyes closed resting EEG recording. The EEG data were collected using self-adhesive electrodes from frontal electrode sites of the International 10/20 system, which included FP1, FP2, AFz, F7, and F8, referenced to linked ears.(Figure) All electrode impedances were below 10 kW. Amplifiers had a band pass filter from 0.5 to 70 Hz (3 dB points). Set-up was accomplished in all cases in less than 5 minutes.
EEG Data Analysis
The device used in this study can compute the TBI-Index in approximately “real-time;” however, to maintain the blinding and perform quality assurance, the TBI-Index was calculated off site. EEG data was subjected to automatic artifact rejection to remove any biologic and non-biologic contamination, such as that from eye movement or muscle movement. An experienced EEG technician also reviewed the selected artifact-free EEG segments for the purpose of confirming data quality for all data analyzed in this study. Previous experience has demonstrated that sufficient artifact-free data (120 seconds) can be obtained from this 10-minute recording.
The artifact-free EEG data from both the algorithm development and test groups to Fast Fourier Transform to extract QEEG features of absolute and relative (%) power, mean frequency, inter- and intra-hemispheric coherence and symmetry computed for the delta (1.5 – 3.5 Hz), theta (3.5 -7.5 Hz), alpha (7.5 to 12.5 Hz), beta (12.5 – 25 Hz) and gamma (30–45 Hz) frequency bands. These measures are described in detail elsewhere.21 All quantitative features to obtain a Gaussian distribution and Z-transformed relative to age-expected normal values. The importance of each of these steps in enhancing the sensitivity and specificity of brain electrical activity has been described in detail elsewhere, as are the robust test-retest reliability and independent replications of the neurometric normative data of brain electrical activity.22,23 Non-linear features of complexity of the electrical signal were also extracted and transformed in the same way.24
Classifier Function
We used the extracted EEG measures described above to develop a discriminat classifier function (biomarker) that maximally separated closed head-injured patients with GSC >8 who were CT+ from those who were CT− patients and controls. We constructed this binary discriminant classification algorithm using iterative methods and cross-validation based on features extracted from all patients in the algorithm development group (n=282).25 Inclusion/exclusion criteria for this population was the same as for the current study as described above and patients were tested in the acute phase (within 24 hours) following injury. The algorithm consists of a multivariate weighted combination of selected linear and nonlinear features of brain electrical activity that mathematically describe the profile of traumatic brain injury statistically most resembling that seen in patients who sustain a closed head injury and are found to be CT+. The result is expressed as a TBI- Index/biomarker ranging from 0–100, where 100 is the highest probability of being CT+. Features that contributed most to this discriminant included: relative power increase in slow waves in frontal regions, relative power decrease in alpha 1 and alpha 2 in frontal regions, power asymmetries in theta and total power between lateral and midline frontal regions, incoherence in slow waves between frontopolar regions and decrease in mean frequency of the total spectrum composited across frontal regions.
Statistical Analyses
The TBI-Index was calculated for the 119 patients in the current study and were not used in the derivation of the index and therefore represents an independent replication/validation of the algorithm. We submitted the brain electrical activity data from all patients in the study to discriminant analysis and obtained a discriminant score. Patients were considered to be positive if the score obtained was greater than or equivalent to a cut-off point derived from the Receiver Operating Curve (sensitivity as a function of specificity) from the original discriminant function. We identified a score of 65 as the point at which 95% of the CT+ population was correctly identified. We calculated the NOC for the CT+ and CT− patients and considered it to be positive if there was a total score of 1 or greater. We also calculated the NOC total score supplemented by the TBI-Index. That is, if either the TBI-Index or the NOC were positive, the classification was considered to be positive. Performance metrics, including sensitivity, specificity, positive predictive value, negative predictive value, and positive and negative likelihood values associated with the independent population in the current study, were then calculated for all measures. In addition, we computed Pearson correlations to assess the relationship between NOC and TBI-Index.
RESULTS
Patient populations
One hundred and nineteen patients met inclusion criteria and were enrolled in this study. The mean age was 48.32 (range 18–92 years) and contained 38 patients (31.9%) with CT+ and 81 (68.1%) with CT−. Distribution by gender did not differ across the 2 groups, with the CT+ group containing 57.1% males and the CT− group 60.9% males. The mean age of patients in each group differed, with mean age higher in the CT+ group than those in the CT− group (CT+ = 61.0, range of 21–92 years; and CT− = 45.0, range of 18–82 years, p < 0.001). It is important to point out that patient age was taken into account prior to calculation of the brain state discriminant index, since all EEG features were age-regressed prior to inclusion in discriminant analyses. The total patient population was enrolled during a 36-month time window. The most common reasons for exclusion of patients for study were acute intoxication (too obtunded to participate), co-morbid diagnosis of dementia, or a non-acute or incidental CT finding (it is estimated that this represents approximately 15%).
Using the Marshall score, 32 of 38 CT+ patients received a score of 2, 1 a score of 3, 1 a score of 4 and 4 a score of 5. CT+ findings included: 60% traumatic hemorrhages (majority being subarachnoid), 29% subdural and epidural hematomas, 8% contusions, 3% other. The majority of the CT− patients received a diagnosis of concussion.
New Orleans Criteria (NOC) Classification
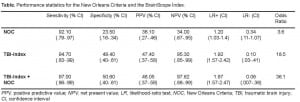
CT+ and CT− patients were classified using a NOC total score of greater than or equal to 1. Using this cut point 35/38 CT+ and 62/81 CT− patients received a positive classification. This resulted in sensitivity of 92.1% (95% confidence interval (CI) = 0.79 to 0.97), and a specificity of 23.5% (CI = 0.16 to 0.34), positive predictive power (PPV) = 36.1% (CI = 0.27 to 0.46), negative predictive power (NPV) = 86.4% (CI = 0.67 to 0.95), a positive likelihood ratio (LR+) = 1.2 (CI = 1.03 to 1.40), and a negative likelihood ratio (LR+) = 0.34 (CI = 0.11 to 1.07) [Table].
TBI-Index
A TBI-Index greater than or equal to the cutoff value (a score ≥65) was used to classify each of the CT+ and CT− patients. A total of 36 of 38 CT + and 40 of 81 CT− patients had TBI-Index greater than or equal to this value. Sensitivity was 94.7% (CI= 0.83 to 0.99), specificity was 50.6% (CI= 0.40 to 0.61), PPV = 47.4% (CI = 0.37 to 0.58), NPV = 95.3% (CI = 0.85 to 0.99), LR+ was 1.92 (CI = 1.57 to 2.42), and LR- was 0.10 (CI= 0.03 to 0.41) [Table 1]. There was also evidence that the TBI-Index was sensitive to the degree of injury within our sample of mTBI patients since the Pearson correlation between the NOC total score and the TBI-Index was found to be +.33, with p < .0001.
New Orleans Total plus TBI-Index
We also classified all patients using the TBI-Index to supplement the NOC total score. A patient was classified as “Combined+” if the NOC total score was 1 or greater or the TBI-Index was greater than or equal to the cutoff value, with a patient classified as “Combined-” if the NOC total score was zero or the TBI-Index was less than the cutoff value. Using this algorithm, 37 of 38 CT + and 41 of 81 CT− patients were correctly classified. Thus, sensitivity was 97.4% (CI= 0.86 to 0.99), specificity was 50.6% (CI = 0.40 to 0.61), PPV = 48.0% (CI = 0.37 to 0.59), NPV = 97.6% (CI = 0.88 to 0.99), LR+ was 1.97 (CI= 1.57 to 2.47), and LR- was 0.06 (95% CI=0.007 to 0.36) [Table 1].
DISCUSSION
In this study all EEG data was collected from a limited montage, with electrodes placed over frontopolar, frontal midline and dorsolateral frontal regions on the forehead. We The rationale for these electrode locations on the published reports that after minor closed head injury the frontal and frontotemporal regions are particularly susceptible/vulnerable to injury, and more likely to be affected than other cortical regions.26–28 This increased susceptibility of the frontal regions most likely results from direct impact of this region and subsequent disruption of the extensive connections between this region and other cortical regions.29 The ability to focus on the frontal regions enhanced the practicality of EEG set-up and use in the ED while not compromising the ability to detect brain dysfunction following closed head injury. A recently published study demonstrated the ability to use these methods in the ED setting, with set-up completed in less than 5 minutes and data acquired in less than 10 minutes.17 As noted above, although for purposes of this study we computed results off-site, in actuality data analysis and computation of the TBI-Index can be performed in “real-time” on the device, again supporting feasibility in the ED environment.
The QEEG-derived TBI-Index appears to be a sensitive measure of brain function that may be used in conjunction with other clinical information to determine whether or not a patient presenting to the ED has a brain injury severe enough to warrant further diagnostic evaluation and treatment. It is of note that the 2 CT+ patients with an index below the cut point (<65) each had a score of 2 on the Marshall CT-scoring criteria, and were discharged from the hospital without intervention. One CT showed a small subarachnoid bleed, (SAH) in the left frontal region without any mass effect with a TBI-Index = 34, with a positive NOC; and the second, a small SAH in the left temporal/parietal region without mass effect and a TBI-Index = 56, with a negative NOC.
The finding that the TBI-Index was greater than the cut point for 49.4% of the CT− patients may indicate that a subset of the CT− patients showed signs of disturbed brain function in the presence of normal brain structure, possibly representing the effects of concussion. Evidence for this hypothesis can be found in a recent publication that used an EEG-based index to document the presence of concussion in college and high school athletes.18,19 These studies noted that the index remained abnormal well past the period when clinical recovery was reported. Also of importance is the finding that 50.6% of the CT− population obtained scores below the cut point, suggesting the lack of structural brain damage in this group, potentially aiding in their screening for CT. Bazarian et al.30reported that after concussion the presence of a normal CT does not rule out the presence of a functional brain injury due to axonal damage. Such concern extends to possible “second impact syndrome,” in cases where the individual may be at risk when returned to play prematurely.31 Derived QEEG indices may reveal signs of brain injury in concussed individuals that are missed by other less objective assessment tools and may play a role in assessing and monitoring residual brain dysfunction in mTBI patients.32This subset of CT− patients will more than likely warrant rapid referral for treatment and counseling as they may represent the population at risk for Post-Concussion Syndrome.
In our sample the CT+ patients were older than those in the CT− group. This almost certainly reflects the inherent increased risk of serious injuries from head trauma in this age group and emphasizes the importance placed on age in determining the severity of mTBI by the Canadian and NOC and the clinical policy statements issued by the CDC.33The resilience of the QEEG method described above to age effects, due to age regression (comparing the patient to age-expected normal values) further emphasizes the clinical use of the method.
In the present population the NOC score for head injury was not as useful for distinguishing the CT + from the CT− patients since specificity was only 23.5%. While 35/38 CT+ patients were identified, 62/81 CT− patients also met criteria. Similar findings to those reported here for the NOC were reported in 2 studies that compared the NOC with the Canadian CT Head Rule using very large populations of mTBI patients.34,35While these studies reported sensitivity for the NOC identification of a neurosurgical lesion or an intracranial injury to be high, they also reported very low specificity values for the NOC (3.0%–12.7%). Since the majority of patients in our sample had mild traumatic brain injury, as verified by subsequent scoring of their CT+ using the Marshall criteria (84.2% had a score of 2), it would appear that the TBI-Index is a more clinically useful index than the NOC within this population since sensitivity was slightly greater and specificity more than doubled. It was noted that 22 patients classified as “high risk” on the NOC were not considered so on the TBI-Index, suggesting that these patients might have been spared CT examinations. Further, it was found that adding the TBI-Index to the NOC total score resulted in increased specificity and more reliable positive and negative likelihood results.
A study of 381 mild head injury patients all of whom received a CT revealed an incidence of 38% positive scans requiring further treatment, a finding consistent with that seen in our patient sample. Age, mode of injury, loss of consciousness, seizures, ENT bleeding, and vomiting did not predict positive CT, while GCS, the presence of focal neurological signs, and the presence of a radiographic skull fracture only had moderate predictive power of a CT+.36 While CTs are readily available in this country recent studies have highlighted the adverse effects of radiation from CT and the fact that increased use increases the individual risk for cancer and overuse in general can increase the incidence of cancer in the population at large. 36,37 In addition, it has been proposed that objective indices of cerebral physiology are necessary to follow the course of recovery and the effectiveness of rehabilitation efforts. We would add that measures of cerebral physiology may be useful for the documentation of the extent of brain dysfunction at the time of injury. These concerns point to the need for biologic markers indicating which patients may recover. This study, if replicated, would suggest that the TBI-Index can play an important role in the ED setting in determining which patients presenting with mTBI require further evaluation.
LIMITATIONS
The sample size for this study was moderate and the authors are aware of the need for prospective independent replications of this work in larger populations with more refined scoring of CT results. Although the inclusion criteria were used to enroll a low-risk group for intracranial hemorrhage, we enrolled a rather high percentage of patients with a positive CT. This high positive CT rate may be partially due to the fact that as a study entry criterion the patient needed to undergo a CT and therefore the very low-risk group was eliminated. The most common reasons for exclusion of patients include: acute intoxication (too obtunded to participate), comorbid diagnosis of dementia, pregnancy, or a non-acute or incidental CT finding (it is estimated that this represents approximately 15%). While the exclusion criteria may limit the immediate applicability of our findings to the general ED population they were applied in order to examine the physiological consequence of mild head trauma in the absence of confounding variables. Future studies will examine how a derived EEG index is changed by these factors. The possibility of spectrum bias due to inclusion/exclusion criteria cannot be eliminated, although it is noted that this would apply to both the TBI-Index and the NOC groups. We did not acquire long-term follow up or neuropsychological testing of patients after ED discharge. In the future this may help to differentiate those patients at high risk for neurological dysfunction, neurocognitive deficits or post-concussive syndrome.
CONCLUSION
In patients presenting to the ED with mTBI, the TBI-Index used in this study had sensitivity levels equivalent to the NOC and specificity that outperformed the NOC (50.6% compared with 23.5%). Combining the index with NOC resulted in a sensitivity of 97.0% (only 1 false negative). This study demonstrates that the hand-held device measuring brain electrical activity can be used in the ED setting and suggests a role in the initial screening of mild traumatic brain-injured patients. Further validation of this TBI-Index is necessary in a consecutive ED sample with common behavioral confounders, as well as its real-time use and incorporation into clinical decisions in the ED.
Footnotes
This research was supported in part by funding from BrainScope, Co., Inc., which covered expenses related to data acquisition. The authors acknowledge the contribution of the research assistants at all clinical sites. EEG data was collected on the BrainScope device in development.
Leslie S. Prichep is a scientific consultant to BrainScope Co., Inc., who provided the funds for this research. Robert Chabot is a research consultant to BrainScope Co., Inc., and provided scientific and statistical expertise. Rosanne Naunheim discloses BrainScope Co., Inc., provides a clinical coordinator. Brian O’Neil discloses BrainScope sponsored the study covering technical costs. No other financial relationships were present.
Supervising Section Editor: John Sarko, MD
Submission history: Submitted May 31, 2011; Revision received October 10, 2011; Accepted December 19, 2011
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.12.6815
Address for Correspondence: Leslie S. Prichep, PhD, Brain Research Laboratories, Old Bellevue Administrative Bldg., 8th Floor 462 First Avenue, New York, NY 10016
Email: leslie.prichep@nyumc.org
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Jager TE, Weiss HB, Coben JH, et al. Traumatic brain injuries evaluated in U.S. emergency departments, 1992–1994. Acad Emerg Med. 2000;7:134–40. [PubMed]
2. Rutland- Brown W, Langlois JAS, Thomas KEM, et al. Incidence of Traumatic Brain Injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–8. [PubMed]
3. Semelka RC, Armao DM, Elias J, Jr, et al. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging.2007;25:900–9. [PubMed]
4. Haydel MJ, Preston CA, Mills TJ, et al. Indications for Computed Tomography in Patients with Minor Head Injury. N Engl J Med. 2000;343:100–5. [PubMed]
5. Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. The Lancet. 2001;357:1391–6.
6. Stein SC, Fabbri A, Servadei F, et al. A Critical Comparison of Clinical Decision Instruments for Computed Tomographic Scanning in Mild Closed Traumatic Brain Injury in Adolescents and Adults. Ann Emerg Med. 2009;53:180–8. [PubMed]
7. Tebano MT, Cameroni M, Gallozzi G, et al. EEG spectral analysis after minor head injury in man. Electroencephalogr Clin Neurophysiol. 1988;70:185–9. [PubMed]
8. Thatcher RW, Walker RA, Gerson I, et al. EEG discriminant analyses of mild head trauma. EEG Clin Neurophysiol. 1989;73:94–106.
9. Thornton KE. Exploratory investigation into mild brain injury and discriminant analysis with high frequency bands (32–64 Hz) Brain Inj. 1999;13:477–88. [PubMed]
10. Thatcher RW, North DM, Curtin RT, et al. An EEG severity index of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2001;13:77–81. [PubMed]
11. Chen XP, Tao LY, Chen AC. Electroencephalogram and evoked potential parameters examined in Chinese mild head injury patients for forensic medicine. Neuroscience Bulletin. 2006;22:165–70. [PubMed]
12. Duff J. The Usefulness of Quantitative EEG (QEEG) and Neurotherapy in the Assessment and Treatment of Post-Concussion Syndrome. Clinical EEG and Neuroscience.2004;35
13. Watson MR, Fenton GW, McClelland RJ, et al. The post-concussional state: neurophysiological aspects. Br J Psychiatry. 1995;167:514–21. [PubMed]
14. Trudeau DL, Anderson J, Hansen L, et al. Findings of mild traumatic brain injury in combat veterans with PTSD and a history of blast concussion. J Neuropsychiatry Clin Neurosci. 1998;10:308–13. [PubMed]
15. Cao C, Tutwiler RI, Slobounov S. Automatic Classification of Athletes With Residual Functional Deficits Following Concussion by Means of EEG Signal Using Support Vector Machine. Neural Systems and Rehabilitation Engineering. 2008;16:327–35. [PubMed]
16. Povlishock JT, Katz DI. Update of Neuropathology and Neurological Recovery After Traumatic Brain Injury. J Head Trauma Rehabil. 2005;20
17. Naunheim RS, Treaster M, English J, et al. Use of Brain Electrical Activity to Quantify Traumatic Brain Injury in the Emergency Department. Brain Inj. 2010;24:1324–9.[PubMed]
18. McCrea M, Prichep LS, Powell MR, et al. Acute Effects and Recovery After Sport-Related Concussion: A Neurocognitive and Quantitative Brain Electrical Activity Study. J Head Trauma Rehabil. 2010;25:283–92. [PubMed]
19. Barr WB, Prichep LS, Chabot RJ, et al. Measuring brain eletrical activity to track recovery from sport related concussion. Brain Inj. In press.
20. Marshall LF, Marshall SB, Klauber M, et al. The diagnosis of head injury requires a classification based on computed axial tomography. Journal of Neurotrauma.1992;9:S287–S292. [PubMed]
21. John ER, Prichep LS, Friedman J, et al. Neurometrics: Computer-assisted differential diagnosis of brain dysfunctions. Science. 1988;293:162–9. [PubMed]
22. Prichep LS. Use of normative databases and statistical methods in demonstrating clinical utility of QEEG: Importance and cautions. Clinical EEG. 2005;36:82–7.
23. Kondacs A, Szabo M. Long-term intra-individual variability of the background EEG in normals. Clinical Neurophysiology. 1999;110:1708. [PubMed]
24. Thakor NV, Tong S. Advances In Quantitative Electroencephalogram Analysis Methods. Annu Rev Biomed Eng. 2004;6:453–95. [PubMed]
25. Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection. International Conference on Artificial Intelligence; 1995.
26. Levin H, Williams D, Eisenberg H, et al. Serial MRI and neurobehavioural findings after mild to moderate closed head injury. Journal of Neurology, Neurosurgery & Psychiatry. 1992;55:255–62.
27. Levin H, Kraus MF. The frontal lobes and traumatic brain injury. J Neuropsychiatry Clin Neurosci. 1994;6:443–54. [PubMed]
28. Ptito A, Chen JK, Johnston KM. Contributions of functional Magnetic Resonance Imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation. 2007;22:217–27.[PubMed]
29. Malloy PF, Aloia M. Frontal Lobe Dysfunction in Traumatic Brain Injury. Seminars in Clinical Neuropsychiatry. 1998;3:186–94. [PubMed]
30. Bazarian JJ, Blyth B, Cincotti F. Bench to Bedside: Evidence for Brain Injury after Concussion– Looking beyond the Computed Tomography Scan. Acad Emerg Med.2006;13:199–214. [PubMed]
31. Bey T, Ostick B. Second Impact Syndrome. WestJEM. 2009;10:6–10. [PMC free article][PubMed]
32. Slobounov S, Cao C, Sebastianelli W. Differential effect of first versus second concussive episodes on wavelet information quality of EEG. Clinical Neurophysiology.2009;120:862–7. [PMC free article] [PubMed]
33. Jagoda AS, Bazarian JJ, Bruns JJ, Jr, et al. Clinical Policy: Neuroimaging and Decisionmaking in Adult Mild Traumatic Brain Injury in the Acute Setting. Ann Emerg Med. 2008;52:714–48. [PubMed]
34. Smits M, Dippel DWJ, de Haan GG, et al. External Validation of the Canadian CT Head Rule and the New Orleans Criteria for CT Scanning in Patients With Minor Head Injury.JAMA. 2005;294:1519–25. [PubMed]
35. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in Patients With Minor Head Injury. JAMA. 2005;294:1511–8.[PubMed]
36. Redberg RF. Cancer Risks and Radiation Exposure From Computed Tomographic Scans: How Can We Be Sure That the Benefits Outweigh the Risks? Archives of Internal Medicine. 2009;169:2049–50. [PubMed]
37. Smith-Bindman R, Lipson J, Marcus R, et al. Radiation Dose ssociated With Common Computed Tomography Examinations and the Associated Lifetime Attributable Risk of Cancer. Archives of Internal Medicine. 2009;169:2078–86. [PubMed]