| Author | Affiliation |
|---|---|
| Michael Loughren, CRNA, PhD | Department of Anesthesia & Operative Services, Madigan Army Medical Center, Tacoma, Washington |
| Sarah Banks, CRNA, MS | Department of Anesthesia & Operative Services, Madigan Army Medical Center, Tacoma, Washington |
| Carleo Naluan, CRNA, MS | Department of Anesthesia & Operative Services, Madigan Army Medical Center, Tacoma, Washington |
| Paul Portenlanger, CRNA, MS | Department of Anesthesia & Operative Services, Madigan Army Medical Center, Tacoma, Washington |
| Arthur Wendorf, CRNA, MS | Department of Anesthesia & Operative Services, Madigan Army Medical Center, Tacoma, Washington |
| Don Johnson, RN, PhD | Department of Anesthesia & Operative Services, Madigan Army Medical Center, Tacoma, Washington |
Introduction
Methods
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
The intraosseous (IO) route has become a popular method to gain access to the peripheral circulation in emergency situations. Despite little supporting data, it is generally believed that IO absorption is immediate and equivalent to the intravenous (IV) route. It is important to determine if rocuronium can effectively be administered by the IO route. The aim of the study was to determine and compare the onset and duration of rocuronium when administered via the IO and IV routes in a normovolemic pig model.
Methods
We recorded electromyographic (EMG) data following tibial IO and peripheral IV administration of rocuronium (1.2 mg/kg) in 10 swine weighing between 56 and 71 Kg. We transformed data were transformed to percent of baseline, determined onset and recovery characteristics.
Results
The onset EMG-time profiles for IO and IV administration were very similar: tibial IO compared to IV administration did not statistically alter the onset of paralysis. The IO group took statistically longer than the IV group to return to 50 (p=0.042), 75 (p=0.034) and 95 (p=0.036) percent of baseline activity.
Conclusion
The duration of effect is statistically longer after IO administration but is more of an academic interest than a clinical concern. The results of this study suggest that rocuronium can effectively be administered via the IO route without the need for dose adjustments.
INTRODUCTION
Intraosseous (IO) access was first contemplated in 1922 by Drinker while examining the marrow cavity of the sternum. The route became popular in the 1940s, and the sternal puncture kit for bone marrow infusions was a common component of emergency medical supplies during World War II.1,2 Following World War II, the use of IO devices began to diminish and was all but abandoned with the rapid development of plastic catheters and routine venous cannulation. In the 1980s, the technique was re-introduced in response to the need for immediate vascular access during cardiopulmonary resuscitation (CPR). Currently, the technique is used throughout the United States and is recognized as an accepted alternative to intravenous (IV) access in pediatric emergencies and, increasingly, in neonatal and adult emergencies.3–5 In addition, the ease, effectiveness, and safety of the technique have led to its use for prehospital and combat emergency care.2,6–8 In current military operations, the use of IO access is being used from the medic at the point of injury to the emergency department at combat hospitals. Despite few data, it is generally believed that IO absorption is immediate and equivalent to the IV route. Pharmacokinetic evaluation of medications commonly used via the IO route is necessary as dose adjustments may be needed to achieve the desired clinical effect. One such medication, rocuronium, a nondepolarizing neuromuscular blocker is used because of its rapid onset to facilitate endotracheal intubation in emergency situations where an IO catheter may be the only access to the vascular system. Information on the onset, duration, and optimal dose of IO rocuronium is beneficial to anesthesia and emergency medical providers.
METHODS
This study was a prospective, between subjects, experimental design. The aim of the study was to determine and compare the onset and duration of rocuronium when administered via the IO and IV routes in a normovolemic pig model. The study protocol was approved by the local Institutional Animal Care and Use Committee. The animals received care in compliance with the Animal Welfare Act and the Guide for the Use of Laboratory Animals.
We observed 10 Yorkshire-cross swine weighing between 56 and 71 Kg for 3 days to ensure a good state of health. They were fed a standard diet and restricted to nothing by mouth (NPO) after midnight the day of the experiment. On the day of the experiment, we randomly assigned them (5 per group) to either the IO or IV groups. We induced general anesthesia with an intramuscular injection of tiletamine and zolazepam (4–8 mg/kg) and then orally intubated the animals. Anesthesia was maintained with isoflurane (1%). We monitored physiologic variables throughout the experiment, and the temperature of the pig was maintained at greater than 36.0 degrees Celsius with the use of a forced air warmer as needed. We obtained electromyographic (EMG) data following a modified method previously described by Shi et al.9 Specifically, an incision was made in the jugular furrow exposing branches of the vagus nerve, which innervate the sternomastoid muscle. We placed 2 subdermal needle electrodes (Medtronic USA, Jacksonville, FL) in the sternomastoid muscle for direct EMG recording. These electrodes were connected to a Nerve Integrity Monitor (NIM)-response 3.0 monitor (Medtronic USA, Jacksonville, FL). We used a Prass monopolar probe (Medtronic USA, Jacksonville, FL) in direct contact with a branch of the vagus nerve for stimulation of the sternomastoid muscle. The stimuli were 0.1 millisecound in duration, 5 mA in intensity (supramaximal) and repeated at 4 Hz. The NIM-response 3.0 monitor was set to run with a 50 millisecond time window with an amplitude scale set at 0.2 mV/division. Event capture was activated at a threshold of 0.02 mV. Once baseline EMG amplitudes were established, the investigators administered 1.2 mg/kg of rocuronium (APP Pharmaceuticals, Schaumburg, IL) in a single bolus either via a 20gauge IV catheter placed in an ear vein or a 15 gauge IO needle (Vidacare Corporation, San Antonio, TX) inserted in the proximal area of the tibia followed by a 10 mL normal saline flush. We confirmed IO placement prior to drug administration with aspiration of bone marrow and easy irrigation of 10 mL of normal saline. EMG amplitudes were measured at baseline and at 15 second intervals until termination of EMG activity. We then measured EMG activity every 5 minutes for the next 90 minutes or until there was a return to baseline values.
We transformed the initial data to percent of baseline and graphed as percent baseline versus time. Individual onset and recovery data were plotted using Excel (Microsoft, Redmond, WA). We used the square of the correlation coefficient (R2) to guide the line of best fit. The line of best fit was used to determine the time from the end of injection to 90% reduction of baseline EMG activity (Onset90), the time to maximum reduction (Onsetpeak), and the maximum reduction of the neuromuscular response (peak effect).10 The time from the end of injection to the return of 25%, 50%, 75% and 95% of baseline EMG activity was also determined from the line of best fit and used to characterize recovery of neuromuscular function.11
The results are expressed as mean ± standard deviation and range. We compared the Onset90 and Onsetpeak of the IO and IV groups using a MANOVA. A repeated ANOVA was used to determine recovery of muscle paralysis with a with a post-hoc least significant difference test. An alpha of 0.05 was used for significance. We performed all analyses with SPSS version 18 (SPSS Inc, Chicago, Illinois).
RESULTS
The weights of the animals were not statistically different by group (p=0.47). Onset data for one subject in the IV group could not be collected because of technical reasons. Baseline EMG amplitudes ranged from 1.0 to 5.6 mV, and all animals achieved a 100 percent reduction in EMG activity.
Onset
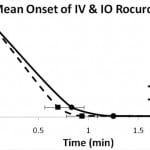
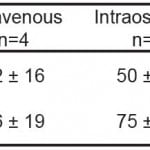
EMG amplitudes in both groups declined rapidly after rocuronium administration. The time from the end of injection to 90% reduction of baseline EMG activity (Onset90) and the time to maximum reduction (Onsetpeak) were determined and used to characterize the onset of neuromuscular blockade. The mean time to Onset 90 was 50 seconds in the IO group compared to 42 seconds in the IV group (p=0.24). The mean time to Onsetpeak was 75 seconds in the IO group compared to 56 seconds in the IV group (p=0.10). The results are summarized in Table 1. Figure 1 shows the initial time course of EMG recordings for IV and IO administration. Tibial IO administration did not statistically alter the onset of paralysis.
Figure 1. Time course to 90 and 100% reduction in baseline EMG following 1.2 mg/kg intravenous (IV) and intraosseous (IO) rocuronium (mean ± SEM).
Table 1. Onset of muscle paralysis following 1.2 mg/kg rocuronium (mean ± SD).
Recovery
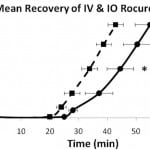
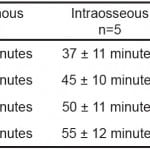
Recovery from neuromuscular blockade was characterized by the time from injection to return of 25, 50, 75 and 95 percent of baseline EMG activity. Figure 2 illustrates the time course of mean EMG activity for the IV and IO groups. Both groups had a rapid and complete response from the rocuronium bolus and began to recover between 20 and 30 minutes following administration. The mean time for the IV group to recovery was faster than the IO group (p<0.05). The IO group took statistically longer than the IV group to return to 50, 75 and 95 percent of baseline activity. Table 2 presents a comparison of the mean times from injection to recovery of these baseline values.
Figure 2. Time course to recovery of 25, 50, 75 and 95% of baseline EMG following 1.2 mg/kg intravenous (IV) and intraosseous (IO) rocuronium (mean ± SEM). *p<0.05
Table 2. Recovery of muscle paralysis following 1.2 mg/kg rocuronium (mean ± SD).
DISCUSSION
There are physiologic differences between the IO and IV routes that could theoretically lead to pharmacokinetics differences. It is generally thought that the most successful sites for IO infusion are long bones that have red marrow because of the richly vascular red marrow cavity. However, the red marrow of the long bones is gradually replaced by less vascular yellow marrow after age five. Yellow marrow contains approximately 95% fat cells and 5% nonfat cells, whereas red marrow contains 60% hematopoietic cells and 40% fat cells.12 In 1990, Fiser recommended that tibial IO only be used in children because of this change of red to yellow marrow with age.13,14 However, yellow marrow contains numerous venous sinusoids that can support modest IO infusion rates. In fact, the tibia and humerus are popular sites for IO access in adults, despite containing almost exclusively yellow marrow after 18 years of age.12 In theory, a drug administered by the IO route could distribute to the bone marrow, creating an absorption phase. The bone marrow may act as a depot or reservoir that slowly releases drug into the circulation. The magnitude of this “depot effect” depends on the make-up of the bone marrow and the lipophilicity of the drug administered. Two basic phenomena or variables have potential to alter the pharmacokinetics of drugs delivered by the IO route: distribution to the bone marrow and blood flow to the bone. This study was designed to gain insight into the extent to which distribution into bone marrow affects the onset and duration of rocuronium when delivered via the IO route.
This study has two major findings. First, there was no statistical difference from the time of administration to complete neuromuscular blockade between the IO and IV administration of 1.2 mg/kg of rocuronium (p=0.10). Second, the recovery of neuromuscular function was significantly longer after IO administration (p=0.03). These results lend support to the notion that some portion of the dose immediately distributes to the marrow and is slowly absorbed over time. However, this portion does not appear to be sufficiently large as the time to onset of neuromuscular blockade was unaffected. Specifically, the fraction of the dose that immediately reached the central circulation was sufficiently large to produce neuromuscular blockade as quickly as the 1.2 mg/kg IV dose. These findings are similar to Prete and colleagues who compared the pharmacokinetics of IV, IO, and endotracheal atropine in macaques.15 Although not significantly different (p=0.055), the mean plasma levels of atropine delivered by the IO route were noticeably higher than IV levels 5 minutes after administration (45 versus 20 nmol/L). This same trend was observed by Spivey and colleagues who compared IO and IV administration of diazepam in swine. Diazepam plasma levels were higher after IV for the first 5 minutes following administration but were less than IO for the remainder of the 20-minute experiment.16,17 This trend has not been observed in all studies. For example, Orlowski compared plasma levels of lidocaine and calcium after IV and IO administration in dogs and found the plasma concentration versus time profiles were indistinguishable.18 In the only human subject study that has compared the pharmacokinetics of drugs administered by the 2 routes, Von Hoff found there were no statistically significant differences between the maximum concentrations (Cmax) or the time to maximum concentration (Tmax) after IO (iliac crest) and IV administration of morphine sulfate.19 Overall, the results support the bioequivalence of IO and IV administration of morphine sulfate in adults. However, the volume of distribution was significantly greater in the IO group, a finding that suggests that there may be some distribution in the marrow. This result is surprising because one would predict minimal distribution when administering a fairly hydrophilic molecule such as morphine into the iliac crest, a site that contains mostly red marrow.
Quaternary ammonium compounds such as rocuronium are relatively hydrophilic compounds. It was unexpected and of academic interest that rocuronium appeared to distribute to the bone marrow to an extent that would affect the recovery of a single bolus dose. However, despite being statistically significant, the increased duration of action would probably not have any clinical significance. The clinical scenarios in which rocuronium would be administered via an IO catheter are unlikely to need rapid recovery. Nevertheless, because a hydrophilic drug, such as rocuronium, shows signs of distribution to the bone marrow then other more lipophilic medications may have greater distribution to the marrow when administered via the IO route. The distribution could be significant enough to create an absorption phase, decreasing concentrations of the medication to such an extent that a dose adjustment is necessary to achieve the desired effect.
LIMITATIONS
The results may not be generalizable to humans; however, pigs are very similar in anatomy and physiology and should approximate results with humans. In fact, the tibia of a 70 Kg pig is shorter and may contain less marrow than a human of the same weight. This difference may underestimate any distribution or depot effect. Additionally, the results may not be generalizable to sternal IO administration. The sternum has a smaller marrow volume and is made up of red marrow compared to the adult tibia, which is made up of almost entirely yellow marrow. In theory, if a medication exhibits a distribution phase when given via the IO route, it should be minimized when administered via sternal IO.
CONCLUSION
In summary, IO and IV administrations of rocuronium (1.2 mg/kg) have similar onset characteristics. The duration of effect is statistically longer after IO administration but is more of an academic interest than a clinical concern. Rocuronium can effectively be used via the IO route without the need for dose adjustments.
Footnotes
Address for Correspondence: Michael J Loughren, CRNA, PhD, Department of Anesthesia & Operative Services, Madigan Army Medical Center, Tacoma, WA. Email: mike.loughren@gmail.com. 3 / 2014; 15:241 – 245
Submission history: Revision received October 30, 2012; Submitted January 30, 2013; Accepted September 4, 2013
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Drinker CK, Drinker KR, Lund CC. The circulation in the mammalian bone marrow. Am J Physiol. 1922; 62:1-92.
2. Dubick MA, Holcomb JB. A review of intraosseous vascular access: current status and military application. Mil Med. 2000; 165:552-559.
3. McCarthy G, Buss P. The calcaneum as a site for intraosseous infusion. J Accid Emerg Med. 1998; 15:421.
4. McCarthy G, O’Donnell C, O’Brien M. Successful intraosseous infusion in the critically ill patient does not require a medullary cavity. Resuscitation. 2003; 56:183-186.
5. Ramet J, Clybouw C, Benatar A, et al. Successful use of an intraosseous infusion in an 800 grams preterm infant. Eur J Emerg Med. 1998; 5:327-328.
6. Glaeser PW, Hellmich TR, Szewczuga D, et al. Five-year experience in prehospital intraosseous infusions in children and adults. Ann Emerg Med. 1993; 22:1119-1124.
7. Glaeser PW, Losek JD, Nelson DB, et al. Pediatric intraosseous infusions: impact on vascular access time. Am J Emerg Med. 1988; 6:330-332.
8. Glaeser PW, Losek JD. Intraosseous needles: new and improved. Pediatr Emerg Care. 1988; 4:135-136.
9. Shi Y, Hou V, Tucker A, et al. Changes of extremity and laryngeal muscle electromyographic amplitudes after intravenous administration of vecuronium. Laryngoscope. 2008; 118:2156-2160.
10. Hemmerling TM, Schurr C, Walter S, et al. A new method of monitoring the effect of muscle relaxants on laryngeal muscles using surface laryngeal electromyography. Anesth Analg. 2000; 90:494-497.
11. Gerstner GE, Goldberg LJ. An analysis of mandibular movement trajectories and masticatory muscle EMG activity during drinking in the guinea pig. Brain Res. 1989;479:6-15.
12. Blebea JS, Houseni M, Torigian DA, et al. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med. 2007; 37:185-194.
13. Salassi-Scotter M, Fiser DH. Adoption of intraosseous infusion technique for prehospital pediatric emergency care. Pediatr Emerg Care. 1990; 6:263-265.
14. Fiser DH. Intraosseous infusion. N Engl J Med. 1990; 322:1579-1581.
15. Prete MR, Hannan CJ Jr., Burkle FM Jr. Plasma atropine concentrations via intravenous, endotracheal, and intraosseous administration. Am J Emerg Med. 1987; 5:101-104.
16. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987; 16:752-757.
17. Spivey WH, Unger HD, McNamara RM, et al. The effect of intraosseous sodium bicarbonate on bone in swine. Ann Emerg Med. 1987;16:773-776.
18. Orlowski JP, Porembka DT, Gallagher JM, et al. Comparison study of intraosseous, central intravenous, and peripheral intravenous infusions of emergency drugs. Am J Dis Child. 1990;144:112-117.
19. Von Hoff DD, Kuhn JG, Burris HA, et al. Does intraosseous equal intravenous? A pharmacokinetic study. Am J Emerg Med. 2008;26:31-38.