| Author | Affiliation |
|---|---|
| Brigitte M. Baumann, MD, MSCE | Department of Emergency Medicine, University of Medicine and Dentistry of New Jersey – Robert Wood Johnson Medical School at Camden, NJ |
| Christopher J. Russo, MD | Division of Emergency Medicine, A.I. duPont Hospital for Children, Wilmington, DE Department of Emergency Medicine, St. Christopher’s Hospital for Children, Philadelphia, PA |
| Daniel Pavlik, PA | Department of Emergency Medicine, Our Lady of Lourdes Medical Center, Camden, NJ |
| Tara Cassidy-Smith, MD | Department of Emergency Medicine, University of Medicine and Dentistry of New Jersey – Robert Wood Johnson Medical School at Camden, NJ |
| Naomi Brown, MD | Division of Emergency Medicine Children’s Hospital of Philadelphia, PA |
| Alfred Sacchetti, MD | Department of Emergency Medicine, Our Lady of Lourdes Medical Center, Camden, NJ |
| Lisa M. Capano-Wehrle, MPH | Department of Emergency Medicine, University of Medicine and Dentistry of New Jersey – Robert Wood Johnson Medical School at Camden, NJ |
| Rakesh D. Mistry, MD, MS | Division of Emergency Medicine Children’s Hospital of Philadelphia, PA |
ABSTRACT
Introduction:
To compare the evaluation and management of pediatric cutaneous abscess patients at three different emergency department (ED) settings.
Methods:
We conducted a retrospective cohort study at two academic pediatric hospital EDs, a general academic ED and a community ED in 2007, with random sampling of 100 patients at the three academic EDs and inclusion of 92 patients from the community ED. Eligible patients were ≤18 years who had a cutaneous abscess. We recorded demographics, predisposing conditions, physical exam findings, incision and drainage procedures, therapeutics and final disposition. Laboratory data were reviewed for culture results and antimicrobial sensitivities. For subjects managed as outpatients from the ED, we determined where patients were instructed to follow up and, using electronic medical records, ascertained the proportion of patients who returned to the ED for further management.
Results:
Of 392 subjects, 59% were female and the median age was 7.7 years. Children at academic sites had larger abscesses compared to community patients, (3.5 versus 2.5 cm, p=0.02). Abscess incision and drainage occurred in 225 (57%) children, with the lowest rate at the academic pediatric hospital EDs (51%) despite the relatively larger abscess size. Procedural sedation and the collection of wound cultures were more frequent at the academic pediatric hospital and the general academic EDs. Methicillin-resistant Staphylococcus aureus (MRSA) prevalence did not differ among sites; however, practitioners at the academic pediatric hospital EDs (92%) and the general academic ED (86%) were more likely to initiate empiric MRSA antibiotic therapy than the community site (71%), (p<0.0001). At discharge, children who received care at the community ED were more likely to be given a prescription for a narcotic (23%) and told to return to the ED for ongoing wound care (65%). Of all sites, the community ED also had the highest percentage of follow-up visits (37%).
Conclusion:
Abscess management varied among the three settings, with more conservative antibiotic selection and greater implementation of procedural sedation at academic centers and higher prescription rates for narcotics, self-referrals for ongoing care and patient follow-up visits at the community ED.
INTRODUCTION
Concurrent with the emergence of Methicillin-resistant Staphylococcus aureus (MRSA) as a community pathogen, the incidence of skin and soft tissue infections has rapidly increased over the past decade.1–3 Since many skin and soft tissue infections, in particular skin abscesses, require urgent evaluation for potential surgical management and antibiotic therapy, emergency departments (EDs) receive a large number of visits for these infections, with children comprising a significant proportion of patients.4–9 Since ED care for pediatric patients is often provided in varied settings, ranging from the community setting to academic pediatric EDs, the management of pediatric skin abscesses has the potential to vary greatly based on the site of emergency care.10–12
While evidence-based consensus guidelines can result in improved patient care, few currently exist for the management of cutaneous abscesses.13 This is further complicated by differences in routine care of adult and pediatric cutaneous abscesses, such as wound packing following incision and drainage, which has come into question in pediatric abscesses, and the use of routine wound cultures for bacterial surveillance.14–16 In addition, oligoanalgesia may complicate ED abscess care, particularly in the pediatric population.17,18 A common approach to abscess drainage includes local anesthesia; however, its use is questionable owing to the lower pH within the abscess cavity, which may reduce the effectiveness of lidocaine.14,19 As a result, local anesthesia, systemic analgesics and procedural sedation are variably used in different practice settings, although these differences have not been previously described.18
Given the potential for highly variable clinical management, we initiated this investigation to examine practice patterns for skin abscess across three different emergency care settings: two academic children’s hospital EDs, a general academic ED and a community ED. We sought to compare these differences for three important aspects of care for skin abscesses: (1) procedural and antimicrobial therapies, (2) rates of wound culture and MRSA prevalence and (3) provision of analgesia.
METHODS
Study Design
This was a retrospective cohort study of children who presented to the ED for evaluation of a skin abscess.
Study Setting and Population
Eligible subjects included children ≤18 years, diagnosed with a cutaneous abscess in one of four participating EDs between January 1 and December 31, 2007. Our goal was to focus on children with routine skin abscesses. To this end, we excluded children who presented with an isolated pilonidal or genital abscess, as these infections frequently involve consultation and management by a subspecialist. We also excluded patients with isolated paronychial infections since management is relatively standard, involving a minor drainage procedure and also because the causative organisms are typically oral rather than skin flora. The study was approved by each participating site’s institutional review board.
Of the four participating study centers, The Children’s Hospital of Philadelphia and St. Christopher’s Hospital for Children are dedicated academic, tertiary care pediatric hospitals with an approximate annual ED census of 82,000 and 58,000 respectively. Cooper University Hospital is an academic center that provides care to both adults and children. This center has a nested pediatric ED located within the main (general academic) ED, where approximately 12,500 children are evaluated annually by fellowship-trained pediatric emergency physicians (EP) from 9 AM to 1 AM, and general EPs for the remaining eight hours. Our Lady of Lourdes Hospital, the fourth center, is a community ED, receiving 9,000 pediatric visits per year. Children are cared for by board-certified EPs and nurse practitioners, none of whom are subspecialty trained pediatric healthcare providers.
We acquired study data using computerized billing and data entry systems and identified potential subjects at each participating center using International Classification of Diseases, Ninth Revision codes consistent with skin infections (680.0–686.9). We reviewed medical records to identify patients who presented with a cutaneous abscess. A random selection of 100 patients from each of the three academic centers and 92 abscess patients from the community ED comprised the study cohort. We achieved the random selection of children from the academic centers using a random number generator (http://www.random.org).
Study Protocol
Data Abstraction Incorporated recommended protocols for chart review research, including case selection protocols, abstractor training and monitoring, and the use of standardized data abstraction forms.20 To ensure data quality, each site underwent a training period where 10 patient medical records were abstracted by both the originating site and the primary site. We compared these data forms and discussed and resolved any discrepancies (<1% of all data) before actual data collection was initiated. Each site had a primary data abstractor who was blinded to the goals of this investigation. Demographics, predisposing conditions,1, 21 physical exam findings, incision and drainage procedures, therapeutics (e.g. antibiotics, warm compresses), and final disposition were recorded. Laboratory data were reviewed for culture results and antimicrobial sensitivities. For subjects managed as outpatients from the ED, we determined where patients were instructed to follow up (pediatrician versus ED) and, using electronic medical records, ascertained the proportion of patients who returned to the ED for further management.
Statistical Analysis
Normally distributed data are presented using summary statistics with means ± standard deviation. Non-parametric data are presented as medians with interquartile ranges (IQR). We compared patient characteristics and patient management at three ED settings, academic pediatric EDs, a general academic ED and a community ED. For comparisons of categorical variables, we used Fisher’s exact and χ2 tests, where appropriate. We performed comparisons between the three settings using one-way analysis of variance for parametric data and the Kruskal-Wallis test for non-parametric data. Comparisons between two settings (i.e. the three academic sites versus the community site) were made using Student’s t-test for continuous variables. Significance was set at p ≤0.05. We analyzed data using SPSS version 15.0 (SPSS, Inc., Chicago, Il).
RESULTS
Patient Characteristics
A total of 392 patients were included in this cohort; data were available for all 392 (100%) eligible subjects. The study population consisted of 230 (59%) girls, 186 (47%) black and 90 (23%) Hispanic children. The median age was 7.7 years (IQR: 2 to 15 years), with a bimodal distribution: 103 (26%) children between one and two years and 68 (17%) children 16–17 years. Age differed among sites, with a younger patient population presenting to the children’s hospital EDs (median age 4.8 years; IQR: 1.7 to 13.8 years), older children at the community site (median age 8.4 years; IQR: 2.7 to 16.1 years), and the oldest group at the general academic ED (median age 12.4 years; IQR: 3.4 to 16.6 years), p= 0.007. We present other patient characteristics, including co-morbidities, prior and family history of skin infections, in Table 1.
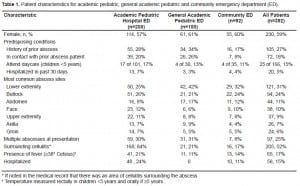
Compared to the community ED, children who presented to centers with dedicated pediatric EPs (the two academic pediatric hospital EDs and the general academic ED) had larger mean abscess size (3.5 ±2.4 cm versus 2.5 ±1.7cm; p=0.02). Over half of subjects, 216 (55%), underwent incision and drainage of their abscess in the ED. Nine additional children, all of whom presented to the academic pediatric hospital EDs, underwent incision and drainage in the operating room. Median age of these patients was 1.4 years (Range: 5 months to 10.5 years). Mean abscess size was smallest in patients who did not undergo incision and drainage (2.8 ±2 cm), larger in those who underwent ED-based procedures (3.5 ±2.4 cm) and largest in those who went to the operating room (5.4 ±3.5cm) (p=0.01). (Table 2).
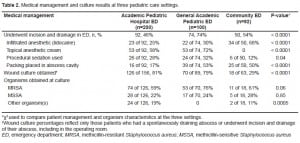
Culture Rates and Antimicrobial Sensitivities
Wound cultures were more commonly obtained at the children’s hospital and general academic EDs. MRSA was the most common organism isolated at all three study sites, ranging between 61–76%. MRSA prevalence did not significantly differ among EDs, nor did the rates of Methicillin-sensitiveStaphylococcus aureus (MSSA) [Table 2]. Of the non-MRSA/MSSA organisms, the most common isolates were coagulase negative Staphylococcus aureus (n=6); Proteus mirabilis (n=5), group A beta hemolytic streptococcus (n=3), and Pseudomonas aeruginosa (n=3).
Antimicrobial sensitivities were obtained for the 138 MRSA isolates. In all three practice settings, there was 100% susceptibility to trimethoprim-sulfamethoxazole and 97–100% susceptibility to vancomycin. Linezolid susceptibility, tested only at the academic pediatric hospital EDs and at the community ED, was 100%. Cindamycin sensitivity was variable: 96% for the academic pediatric hospital EDs, 98% for the general academic ED and 80% for the community ED.
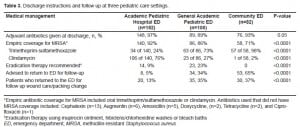
Outpatient Instructions
At discharge, outpatient antibiotics were routinely prescribed at all sites, with practitioners at the academic pediatric hospital EDs prescribing empiric coverage for MRSA in the highest proportion of patients (Table 3). Instructions or prescriptions for eradication therapy were only provided at the academic pediatric hospital and the general academic EDs and included mupirocin ointment (n=35) and chlorhexidine baths (n=20) [Table 3]. Follow-up recommendations also varied by site: academic pediatric hospital EDs were the most likely to refer patients back to their primary care providers. In contrast, the community ED instructed the majority of patients to return to the ED for follow up (Table 3). The highest follow-up rates were at the general academic and the community EDs. Of children who returned for follow up, nine at the two academic pediatric hospital EDs, three at the general academic ED and five at the community ED, had an unchanged or worsening presentation. Based on electronic admission data and subsequent review of individual medical records, 10 patients originally discharged from the ED were admitted to the hospital within 30 days of their initial visit for worsening of their original abscess. One child was admitted to one of the children’s hospitals, six were admitted to the general academic hospital and three were admitted to the community hospital.
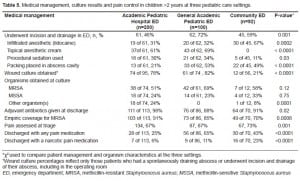
Procedural Sedation and Analgesia
Assessment and treatment of pain differed among study sites. Although the community ED was the least likely to provide procedural sedation for abscess incision and drainage or use a topical anesthetic for pain control, practitioners at the community ED were the most likely to use infiltrated anesthesia prior to incision and drainage and to provide patients with oral pain medication. For the procedural sedation procedures conducted at the three academic centers (academic pediatric hospital and general academic EDs), a narcotic (fentanyl or morphine) was only used in two (2%) [academic pediatric hospital EDs] and 10 (14%) [general academic ED] patients. Ketamine was used in 31 (19%), midazolam in 35 (21%), and propofol in eight (5%) of the three academic center patients. Provision of and instructions for the use of analgesics at discharge also differed between the three settings, with the general academic and the community EDs most frequently providing analgesic instructions and prescriptions (Table 4).

Age as a Factor Influencing Outcomes
Given the relatively younger patient population served by the academic pediatric hospital EDs, we were concerned that this patient subset may have influenced our results. To address this potential confounder, we excluded children two years and under and conducted selected analyses on the older subjects. These findings are presented in Table 5.
DISCUSSION
In this study we describe the current presentation, bacteriology, sedation and analgesia practices and therapeutic strategies for pediatric cutaneous abscesses in three different ED settings. Prior studies of other conditions have demonstrated differences in patient characteristics and specific aspects of medical management between pediatric and general EDs; however, ours is the first to examine practice differences for pediatric abscesses among varying sites of emergency care.22,23
Medical management of patients differed among the academic pediatric hospital, the general academic and the community EDs. Abscess incision and drainage rates were higher in our four sites (55%) than previously described, with the highest rate in the general academic ED.11 Use of packing was nearly 2.5 times higher in the general academic and in the community EDs when compared to the academic pediatric hospital EDs. This difference may be due to provider preference, and neither method is considered superior to the other. Packing is theorized to prevent the wound margins from closing and forming a potential dead space, and periodic removal of packing is considered to provide gentle debridement of necrotic tissue.14 These theoretical benefits, however, have never been scientifically demonstrated, and a recent investigation demonstrated no significant difference in the need for a second intervention in adults who underwent packing of their abscess (17%) compared to those who did not (20%).15 An alternative reason for reduced packing rates at dedicated children’s hospitals is that abscess patients are routinely referred back to their pediatricians for ongoing care. Most pediatricians are not familiar or comfortable with the management of a packed wound, and, upon encountering one, may refer the patient back to the ED for ongoing wound management.
We also demonstrated differences in wound culture rates for pediatric skin abscesses. The academic pediatric hospital EDs and the general academic ED almost routinely obtained wound cultures, whereas the community ED obtained them only one third of the time. The community ED practice is discordant with recommendations from the Center for Disease Control, which encourages clinicians to collect specimens for culture and antimicrobial susceptibility testing from all patients with purulent skin lesions.24 Recently, however, the use of routine culturing has been questioned, with some EPs suggesting that unless the patient appears systemically ill or is immunocompromised, simple incision and drainage without routine antimicrobial susceptibility testing or therapy should suffice.15,16 Two additional concerns regarding all-inclusive routine culturing, are whether it is appropriate to conduct such testing when the results will minimally (or not at all) alter patient management, and, whether it is appropriate for the individual patient to bear the cost of a test for perceived public health benefit.25
Although overall MRSA prevalence was higher in our cohort than previously described, MRSA prevalence in the community ED most closely approximated rates reported in the literature.10,11,26The routine use of antibiotics was somewhat unexpected, since only about half of the patients in our sample had a surrounding cellulitis documented in their medical record, an indication for antimicrobial therapy in the setting of a draining cutaneous abscess.16 There are several reasons why practitioners at the academic pediatric hospital and the general academic EDs were more likely to initiate empiric therapy for MRSA than the practitioners at the community ED. Given the tertiary care centers at which they practice, pediatric specialists are more likely to encounter children at higher risk for complications due to immunosuppression or comorbid factors. Alternatively, the high rates of MRSA prevalence may have influenced adjuvant outpatient therapy. Lastly, pediatric EM healthcare providers may be more likely to encounter life-threatening infections due to community associated-MRSA (CA-MRSA), due to referrals and transfers of such children to their highly specialized practice settings. This may also have lowered their thresholds for administering antibiotics for uncomplicated skin abscesses.27–29
Nevertheless, the high rate of empiric antimicrobial therapy at all three practice settings is not consistent with the standard of care for uncomplicated abscess management, which remains incision and drainage.16 With the emergence of MRSA, this practice was re-examined in an adult population, where over 90% of patients experienced a clinical cure after undergoing incision and drainage and receiving a placebo.30 Similar observations are documented in children who were discharged after their incision and drainage without antibiotics or on agents ineffective against MRSA.12,31,32 The results of a recent randomized controlled trial in children provides additional evidence that antimicrobial therapy is not required for the resolution of uncomplicated abscesses after incision and drainage.33 At three months, there was no difference between placebo and antibiotic treatment with respect to new abscess formation.33 At this time, it is unknown if CA-MRSA-associated life-threatening infections and fatalities are related to prior or current cutaneous abscesses.34 Until this is demonstrated, the routine use of antimicrobial agents for uncomplicated, draining cutaneous abscesses remains questionable.16
We noted differences in the use of procedural sedation and analgesia among the sites of care as well. Topical anesthetics and procedural sedation for abscess drainage were more routinely employed in the academic pediatric hospital and the general academic EDs, whereas lidocaine infiltration was more common at the community site. These differences may be due to several factors. The mean abscess size in the community sample was approximately one centimeter smaller as compared to mean abscess size at the other sites. A smaller abscess may have lent itself to more effective local anesthesia. In contrast, procedural sedation may have been deemed necessary for the larger abscesses which were encountered at the other three sites. At discharge, the provision of analgesics (or instructions for the parent to provide the child with an analgesic medication) was also the lowest at the academic pediatric EDs. In contrast, the community ED had the highest rates of oral narcotic medication use during the ED visit and also at discharge. Our findings demonstrate that the need for acute pain control appears to be recognized across practice settings, yet improvements in pain assessment and outpatient pain management are still needed. These therapeutic oversights suggest that oligoanalgesia in the pediatric abscess population remains an issue at academic pediatric hospital, general academic and community EDs.
LIMITATIONS
Because we identified study subjects using billing data from each site, it is possible that potential subjects were missed, owing to errors in coding. Additionally, all data were obtained via medical record review, thus subjecting the study to information bias from missing data elements. The academic pediatric hospital EDs provided care to children who were younger than those who presented to the general academic and the community EDs, which may have had some influence on practitioners’ evaluation and management of pediatric abscess patients. We respectfully counter that age was not a primary factor in practice patterns, since the differences we noted in the use of procedural sedation and topical anesthetics for drainage procedures, abscess packing, antibiotic administration and selection, the assessment of pain at triage, and recommendations for follow up and for outpatient pain control remained even after limiting the analyses to children over two years of age.
We were also unable to differentiate if MRSA abscesses were from community or hospital-associated strains. Our intention was to be inclusive of routinely evaluated cutaneous abscesses, not to focus on MRSA specifically. Cultures were not obtained in all cases of cutaneous abscesses, so our report of MRSA prevalence rates may not be accurate, although wound cultures were among the practices we wished to assess.
While not a primary objective of this investigation, we did attempt to assess outcomes. These efforts were limited, due to the retrospective nature of this investigation. To maximize our ability to identify adverse outcomes, such as revisits to the ED and hospitalization, we conducted electronic ED visits, admissions and medical record reviews. Given that the four sites were all within a circumscribed geographic area, we are confident that the majority of patients who may have worsened during the 30-day window would have presented to one of the four sites and, therefore, would have been captured by our review. Pain scores were not consistently documented and only available for older children. Thus, the mean pain scores we reported may not accurately represent the level of pain in children at each site. Lastly, there were only four sites that participated in one metropolitan area, with only one general academic pediatric ED and one community ED, limiting the generalizability of our results.
CONCLUSION
The results of our study demonstrate that the management of pediatric skin abscesses vary greatly by site of care. Community ED practitioners are more likely to use local anesthesia and wound packing but less likely to routinely obtain wound cultures. Academic pediatric hospital and general academic EDs, often staffed with pediatric emergency trained physicians, were more likely to use procedural sedation during ED management and send cultures after drainage procedures. Discharge practices also differed between sites. Practitioners at academic pediatric hospital EDs were more likely to refer patients back to their pediatrician, whereas at the community ED, and less so for the general academic ED, patients were instructed to return for ongoing care. Outpatient pain management was highest at the general academic ED and practitioners at the community ED were the most likely to prescribe a narcotic pain reliever. Our findings highlight the differences in abscess management across emergency care settings, and reflect the lack of consensus regarding therapy for pediatric skin abscess. Dissemination of current research findings and subsequent standardization of care across settings could result in consistent and improved care for pediatric abscesses. As best evidence practices continue to develop, translation of this knowledge should be targeted at academic children’s hospital, general academic, and community EDs.
Footnotes
Supervising Section Editor: Sukhjit S. Takhar, MD
Submission history: Submitted May 3, 2010; Revision received August 27, 2010; Accepted September 17, 2010
Reprints available through open access at http://escholarship.org/uc/uciem_westjem.
Address for Correspondence: Brigitte M. Baumann, MD, MSCE
Department of Emergency Medicine, One Cooper Plaza, Camden, NJ 08103
Email: baumann-b@cooperhealth.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Emergency ID net study group. Methicillin-resistantS. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–74. 17; [PubMed]
2. Frazee BW, Lynn J, Charlebois ED, et al. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45(3):311–20. [PubMed]
3. Zaoutis TE, Toltzis P, Chu J, et al. Clinical and molecular epidemiology of community-acquired methicillin-resistant Staphylococcus aureus infections among children with risk factors for health care-associated infection: 2001–2003. Pediatr Infect Dis J. 2006;25(4):343–8. [PubMed]
4. Pallin DJ, Egan DJ, Pelletier AJ, et al. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–8. [PubMed]
5. Hersh AL, Chambers HF, Maselli JH, et al. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168(14):1585–91. [PubMed]
6. Ochoa TJ, Mohr J, Wanger A, et al. Community-associated methicillin-resistant Staphylococcus aureus in pediatric patients. Emerg Infect Dis. 2005;11(6):966–8. [PMC free article] [PubMed]
7. Purcell K, Fergie J. Epidemic of community-acquired methicillinresistant Staphylococcus aureusinfections: a 14-year study at Driscoll Children’s Hospital. Arch Pediatr Adolesc Med.2005;159(10):980–5. [PubMed]
8. Buckingham SC, McDougal LK, Cathey LD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus at a Memphis, Tennessee Children’s Hospital. Pediatr Infect Dis J.2004;23(7):619–24. [PubMed]
9. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–44. [PubMed]
10. Kairam N, Silverman ME, Salo DF, et al. Cutaneous Methicillinresistant Staphylococcus aureus in a Suburban Community Hospital Pediatric Emergency Department. J Emerg Med. (in press).
11. Hasty MB, Klasner A, Kness S, et al. Cutaneous community-associated methicillin-resistantStaphylococcus aureus among all skin and soft-tissue infections in two geographically distant pediatric emergency departments. Acad Emerg Med. 2007;14(1):35–40. [PubMed]
12. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by communityacquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23(2):123–7. [PubMed]
13. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis.2005;41(10):1373–406. [PubMed]
14. Pollack CV. Cutaneous and subcutaneous abscesses. In: Singer AJ, Hollander JE, editors.Lacerations and Acute Wounds: An Evidence-based Guide. Philadelphia, PA: F.A. Davis Company; 2003. p. 166.
15. O’Malley GF, Dominici P, Giraldo P, et al. Routine packing of simple cutaneous abscesses is painful and probably unnecessary. Acad Emerg Med. 2009;16(5):470–3. [PubMed]
16. Gorwitz RJ. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and update. Pediatr Infect Dis J. 2008;27(10):925–6. [PubMed]
17. Rupp T, Delaney KA. Inadequate analgesia in emergency medicine. Ann Emerg Med.2004;43(4):494–503. [PubMed]
18. MacLean S, Obispo J, Young KD. The gap between pediatric emergency department procedural pain management treatments available and actual practice. Pediatr Emerg Care. 2007;23(2):87–93.[PubMed]
19. Khalil PN, Brand D, Siebeck M, et al. Aspiration and injection-based technique for incision and drainage of a sacrococcygeal pilonidal abscess. J Emerg Med. 2009;36(1):60–3. [PubMed]
20. Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27:305–8. [PubMed]
21. Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquiredStaphylococcus aureus infections in children. Clin Infect Dis. 2005;40(12):1785–91. [PubMed]
22. Bourgeois FT, Shannon MW. Emergency care for children in pediatric and general emergency departments. Pediatr Emerg Care. 2007;23(2):94–102. [PubMed]
23. Magilner D, Byerly MM, Cline DM. The prevalence of community-acquired methicillin-resistantStaphylococcus aureus (CA-MRSA) in skin abscesses presenting to the pediatric emergency department. N C Med J. 2008;69(5):351–4. [PubMed]
24. Gorwitz RJ, Jernigan DB, Powers JH, Jernigan JA. 2006. , and participants in the CDC convened experts’ meeting on management of MRSA in the community. Strategies for clinical management of MRSA in the community: Summary of an experts’ meeting convened by the Centers for Disease Control and Prevention.
25. Abrahamian FM, Shroff SD. Use of routine wound cultures to evaluate cutaneous abscesses for community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med.2007;50(1):66–7. [PubMed]
26. Sattler CA, Mason EO, Jr, Kaplan SL. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptibleStaphylococcus aureus infection in children. Pediatr Infect Dis J. 2002;21(10):910–7. [PubMed]
27. Young LM, Price CS. Community-acquired methicillin-resistant Staphylococcus aureus emerging as an important cause of necrotizing fasciitis. Surg Infect. 2008;9(4):469–74.
28. Creel AM, Durham SH, Benner KW, et al. Severe invasive community-associated methicillin-resistant Staphylococcus aureus infections in previously healthy children. Pediatr Crit Care Med.2009;10(3):323–7. [PubMed]
29. Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics.2005;115(3):642–8. [PubMed]
30. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother.2007;51(11):4044–8. [PMC free article] [PubMed]
31. Fergie JE, Purcell K. Community-acquired methicillin-resistant Staphylococcus aureus infections in south Texas children. Pediatr Infect Dis J. 2001;20(9):860–3. [PubMed]
32. Hyun DY, Mason EO, Forbes A, et al. Trimethoprim-sulfamethoxazole or clindamycin for treatment of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2009;28(1):57–9. [PubMed]
33. Duong M, Markwell S, Peter J, et al. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med.2010;55(5):401–7. [PubMed]
34. Sherer DM, Dalloul M, Salameh G, et al. Methicillin-resistant Staphylococcus aureus bacteremia and chorioamnionitis after recurrent marsupialization of a bartholin abscess. Obstet Gynecol.2009;114:471–2. [PubMed]


