| Author | Affiliation |
|---|---|
| Paul Tran, MD, MS | University of Nebraska Medical Center, Department of Emergency Medicine, Omaha, NE |
| Eric J.M. Reed, MD | University of Nebraska Medical Center, Department of Emergency Medicine, Omaha, NE |
| Francis Hahn, MD | University of Nebraska Medical Center, Department of Radiology, Omaha, NE |
| Jason E. Lambrecht, MD | University of Nebraska Medical Center, Department of Emergency Medicine, Omaha, NE |
| James C. McClay, MD | University of Nebraska Medical Center, Department of Emergency Medicine, Omaha, NE |
| Matthew F. Omojola, MD | University of Nebraska Medical Center, Department of Radiology, Omaha, NE |
ABSTRACT
Introduction:
Pneumocephalus typically implies a traumatic breach in the meningeal layer or an intracranial gas-producing infection. Unexplained pneumocephalus on a head computed tomography (CT) in an emergency setting often compels emergency physicians to undertake aggressive evaluation and consultation.
Methods:
In this paper, we report three cases of pneumocephalus that appear to result from retrograde injection of air through an intravenous (IV) catheter. We also performed a retrospective study to determine the incidence of presumed IV-induced pneumocephalus and etiologies of pneumocephalus in our emergency department (ED) population.
Results:
The incidence of idiopathic and presumed IV-induced pneumocephalus was 0.034% among all head CTs ordered in the ED and 4.88% among cases of pneumocephalus seen in the ED. These cases are characterized clinically by the absence of signs and symptoms of pathologic pneumocephalus and radiographically by the distribution of air densities along the cranial venous system on head CTs.
Conclusion:
Idiopathic and presumed IV-induced pneumocephalus could be considered in the workup of ED patients with unexplained intracranial air on head CT if there are no findings of pathological causes for the pneumocephalus on history and physical examination and if the head CTs show a characteristic distribution of air limited to the cranial venous system. Knowledge of this clinical entity in the evaluation of ED patients with unexplained pneumocephalus can lead to more efficient emergency care and less patient anxiety.
INTRODUCTION
Pneumocephalus on computed tomography (CT) of the head indicates the presence of air or gas within the cranial vault and implies a breach in the craniodural barrier or an intracranial gas-producing infection. Pathological conditions that can give rise to pneumocephalus include head injury, tumor, barotrauma, and infection, among others. Although rarely encountered, tension pneumocephalus is a clinical emergency in which pneumocephalus produces a mass effect on the brain. In the emergency setting, the identification of pneumocephalus on head CT thus usually compels emergency physicians to pursue aggressive evaluation and specialty consultation. These measures are costly and can add to patient discomfort and anxiety.
Pneumocephalus has been recognized with increasing frequency in medical and emergency medicine literature.1 Our search of the MeSH database in English, using the major heading “pneumocephalus,” yielded 652 articles. Of these, 301 (46.2 %) were published in the last 10 years. Although pneumocephalus is most commonly seen after a neurosurgical procedure and trauma, many case reports have implicated a diverse range of etiologies. We have recently noted occasional cases of pneumocephalus in the emergency department (ED) that lack an obvious medical cause. We report here three cases of pneumocephalus that appear to result from retrograde injection of air through an intravenous (IV) catheter. The lack of signs and symptoms of pathologic causes of pneumocephalus in these cases, combined with distinct radiographic features, can be useful to emergency physicians. We also performed a retrospective study to determine the incidence of idiopathic and presumed IV-induced pneumocephalus and the etiologies of undifferentiated pneumocephalus in our ED population.
METHODS
This is a case series of idiopathic pneumocephalus and retrospective study from May 2005 to May 2007 at an inner-city university ED in the Midwest. The ED, which sees 48,000 patients per year, is a Level 1 trauma center and a major tertiary referral center for neurosurgical services. All neuroradiology studies at this institution are read by one of the two neuroradiologists (MFO and FH), who manually collected the cases of pneumocephalus for this study. At the end of the study period, we performed a computer query using the free-text method. The query searched for all head CTs ordered from the ED between May 2005 and May 2007, using the search terms pneumocephalus, pneumocephali, (intracranial) air, (intracranial) gas, (intracranial) aerocele, (intracranial) pneumocyst, and (intracranial) pneumocele. We performed a free-text search method, examining dictated radiology reports for the above search terms during the study period. Once electronically identified, these reports were manually reviewed and verified against the cases identified by the neuroradiologists. Medical records of pneumocephalus cases were subsequently reviewed, clinical data abstracted, and etiology categorized by a research associate and one of the authors (TPT); patients were not contacted directly. The protocol was approved by the local IRB.
RESULTS
There were 8,747 CTs performed during the 24-month study period. Of these, 68 (0.78%) showed pneumocephalus. Table 1 tabulates the etiologic categories for these 68 cases. We identified three cases of idiopathic pneumocephalus, due presumably to injection of air through IV catheter, for an incidence of 0.034%.
Case 1
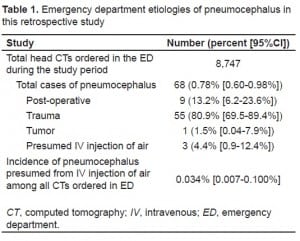
A 55-year-old female presented to the ED with the chief complaint of a frontal headache. A peripheral IV line was placed during the initial management. The initial head CT without contrast in the ED showed pneumocephalus in or surrounding the cavernous sinuses, as well as a small amount of epidural air at the level of the foramen magnum (Figure 1). The patient was admitted to the hospital for observation and neurosurgical consultation, and her hospital course was uneventful. The consensus by the admitting service, neurosurgery, and neuroradiology was that the pneumocephalus was iatrogenic, presumably from IV injection of air. The patient was discharged home the next day. A repeat head CT six days later showed complete resolution of the pneumocephalus. Follow up at four months as an outpatient showed no symptoms related to her pneumocephalus.
Case 2
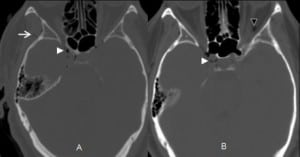
An 87-year old-female presented to the ED with the chief complaint of altered mental status. The family stated that the patient was agitated more than usual, with no history of a recent fall, head trauma, fever, or surgery. A peripheral IV line was placed during the initial management. The initial head CT without contrast in the ED showed gas in the right cavernous sinus and superior ophthalmic vein (Figure 2). The patient was admitted for observation and medical management. The consensus by the admitting service and neuroradiology was that the pneumocephalus was iatrogenic, presumably from IV injection of air. The patient was discharged to an assisted living facility two days later. A follow-up CT scan two months later showed complete resolution of the pneumocephalus.
Case 3
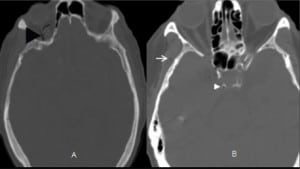
A 56-year-old male presented to the ED with the chief complaint of a fall after experiencing a loss of control of his body. The patient did not suffer any head trauma as a result of the fall. A peripheral IV line was placed during the initial management. The initial head CT without contrast in the ED showed small punctate areas of air within the cavernous sinus (Figure 3). The patient was admitted to the hospital for further workup. The consensus by the admitting service and neuroradiology was that the pneumocephalus was iatrogenic, presumably from IV injection of air. He was discharged home the next day. Follow-up 10 weeks later showed no lasting sequelae from the pneumocephalus.
In all three cases we found no evidence in the nursing documentation that pointed to an identification of witnessed IV infusion of air, malfunction of the IV contrast injection equipment, or one particular individual who might have been responsible for the three cases presented.
DISCUSSION
In this study idiopathic pneumocephalus, presumably from retrograde IV injection of air, is a relatively uncommon event, occurring in approximately one out of 3,000 CT head scans ordered from the ED. As discussed in subsequent sections, the diagnosis of presumed IV-induced pneumocephalus could be considered in the evaluation of unexplained pneumocephalus if 1) the history and physical examination are inconsistent with infection in the head and neck area, craniofacial trauma, barotrauma, or recent cranial surgery; 2) patients do not have the classic symptoms of tension pneumocephalus; and 3) the pattern of air observed on head CT follows the cranial venous anatomical distribution.
Pneumocephalus was first described by Lecat in 1741.2 Thomas revisited the concept in an 1866 autopsy report of a patient who sustained head trauma, and Chiari in 1884 in another autopsy report of a patient with chronic ethmoid sinusitis.3 Chiari’s report was remarkable not only because he was the first to demonstrate the presence of intracranial air during life, but also because he was the first to advance a mechanism for the intracranial air. The force of sneezing, which blew air through a fistula between the ethmoid sinus into the frontal cavity, resulted in the fatal pneumocephalus in this patient. The term pneumocephalus, however, was not officially introduced into the medical vernacular until Wolff’s paper in 1914.3
Pneumocephalus, which literally means “air inside the head,” implies a compromise in the craniodural barrier or the presence of gas-forming infection inside the cranial vault. This breach in the craniodural barrier is most commonly associated with traumatic fractures of the sinuses, skull base, cranial vault or mastoid air cells. While as much as 2 ml of air is required for pneumocephalus to be visible on x-rays, with the advent of CT, as little as 0.5 ml can be detected.4 Depending on the route of entry, air can be seen in the intraventricular, brain parenchymal, subarachnoid or subdural space. Intracranial air that produces a mass effect is known as tension pneumocephalus. This clinical emergency is caused by a ball-valve mechanism that allows air (or anesthetic gas) to enter but not to exit the cranium.3 Pneumocephalus on a head CT usually compels emergency physicians (EP) to pursue a thorough search for its cause.
Numerous case reports on pneumocephalus in the literature in recent years demonstrate a wide range of etiologies. In Markham’s series of 295 cases of pneumocephalus, 73.9% were caused by trauma, 12.9% neoplasm, 8.8% infection, 3.7% post-operative, and 0.6% idiopathic.5 In our review of 68 cases of pneumocephalus seen in the ED, 55 (80.88%) were caused by trauma, one (1.47%) neoplasm, zero (0%) infection, nine (13.24%) post-operative, and three (4.88%) idiopathic, presumed to be from IV injection of air (Table 1). The differences between Markham’s and this case series are largely due to selection bias and detection method. This study was a case series of a heterogeneous ED patient population whose pneumocephalus was detected by CT vs. Markham’s series of 284 cases, whose pneumocephalus was detected by x-rays. Incorporating recent case reports, we have updated and categorized the general etiologies for pneumocephalus (Table 2).

Patients with clinically significant pneumocephalus may present with nausea, vomiting, fever, headache, confusion, agitation, syncope, lethargy, speech changes, aphasia, vision changes (scotoma, double vision, hemianopsia), seizure, paresis/hemiparesis, ataxia, and rhinorrhea.2,6 The evaluation of such patients should focus on signs of infection, level of consciousness, pupillary responses, eye movements, and motor responses.7 A sudden decrease in the patient’s level of consciousness or a lack of a pupillary response would strongly suggest increased intracranial pressure and the need for urgent neurosurgical evaluation. CT of the head is the mainstay of imaging in the workup of such patients.
On a head CT without contrast, pneumocephalus is best seen on a soft tissue window. Air having a Hounsfield unit of approximately −1,000 can be distinguished from fat (−50), water (0–10), and gray/white matter (20–30). The presence of tension pneumocephalus may show the findings of “Mount Fuji” (Figure 4) or “Bubbling Brain.”8,9
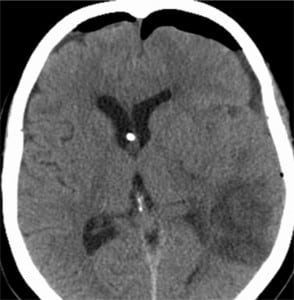
Our review of the case reports in the literature indicates that in the majority of cases, pneumocephalus is treated expectantly.10–14 Since absorption of air occurs in 85% of the cases in the first week, once the source of air is identified or controlled, most patients without significant signs or symptoms of increased intracranial pressure can be observed clinically. Suspicion of tension pneumocephalus warrants urgent medical treatment and neurosurgical consultation as neuronal damage may occur secondary to reduced cerebral perfusion. Nevertheless, expectant therapy may still be appropriate in moderately severe tension pneumocephalus. Hyman reported successful expectant management during the acute phase for a patient who presented in coma from spontaneous tension pneumocephalus.15 Conservative medical management of pneumocephalus includes bed rest, analgesia, head elevation, and avoidance of coughing, sneezing, nose blowing, or the Valsalva maneuver. Additional therapeutic recommendations include laxative use to decrease intra-abdominal pressure during bowel movements and supplemental oxygen therapy to hasten the absorption of pneumocephalus (vs. air).16 Although clinicians have prescribed hyperbaric oxygen therapy (HBO) for treating pneumocephalus, there is currently no literature on HBO efficacy. Hyperosmolar therapy with mannitol can be used as a temporizing medical therapy prior to surgical treatment.17,18 Pneumocephalus and CSF leaks secondary to traumatic dural tears are self limited and do not require prophylactic antibiotics unless there are signs of infection.19–21 Definitive surgical treatment is indicated for significant intracranial hypertension, persistent craniodural leaks, or persistent pneumocephalus lasting longer than one week. In these cases, prophylactic antibiotics are usually recommended, with or without signs of infection, while patients await neurosurgical repair.22
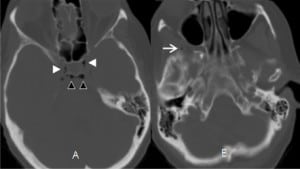
Having considered the standard causes for pneumocephalus, the consensus among the neurosurgical and neuroradiological consultants was that these three cases of idiopathic pneumocephalus occurred as a result of the injection of air into the IV. While infusion from a peripheral IV site in the upper extremities normally travels through the axillary, subclavian, and brachiocephalic veins to empty into the superior vena cava (SVC), the infusion can also flow cephalad through the internal/external jugular veins to the cranial venous system under certain clinical situations (Valsalva maneuvers as in coughing, stenotic brachiocephalic vein, partially obstructed SVC, or low flow state as in heart failure). We have documented CT images of retrograde flow of contrast dye from a peripheral IV site into the internal and external jugular venous system (Figure 5). Since air is lighter than blood, which in turn is lighter than contrast, under similar clinical situations, air bubbles can flow retrograde up through the jugular veins with less difficulty than contrast dye, especially in patients who are in a reclining position (due to the buoyancy force). Once in the jugular veins, air continues its ascension in those patients who are in reclining position to accumulate in the highest areas of the head: the orbital veins, cavernous sinuses, frontal venous system, petrosal sinuses, and the superficial temporal veins. This is what we observed in Figures 1–3.

Clinically, this model explains the clinical response to the expectant management for these three patients. All three had peripheral IVs. None of the three had the typical etiologies for pneumocephalus, such as post-craniofacial procedure, trauma, neoplasm, or infection. Detailed examinations of these three cases revealed no history of congenital septal defects, congenital craniodural defects, or barotrauma. All were treated for idiopathic pneumocephalus, presumably from IV injection of air and improved rapidly, with follow up showing no clinical sequelae of pneumocephalus. Based on these considerations, we propose that the possibility of IV-induced pneumocephalus be considered in the evaluation of unexplained pneumocephalus in the ED, if there is an absence of clinical features associated with trauma, infection, tumor, barotrauma, or tension pneumocephalus and in combination with the presence of characteristic air distribution on head CT (Table 3). Our data and hypothesis for the retrograde reflux of air are consistent with those proposed by Thompson and Liu.23, 24

In this series, the incidence of idiopathic pneumocephalus, presumably from IV injection of air, is approximately 0.034% of the head CTs performed in the ED (with an IV placement). While pneumocephalus usually signifies a serious pathological condition in the emergency setting, recognizing the possibility of pneumocephalus secondary to IV injection of air can help narrow the differential, facilitate care, avoid unnecessary diagnostic tests, interventions, prolonged hospital stays, anxiety, and discomfort for this subset of patients.
LIMITATIONS
The study is limited by its small size and single-site location in the Midwest. The incidence data for pneumocephalus in other EDs may be different, depending on the patterns of local clinical practice. We have offered no definitive proof for the retrograde flow of air through the IV catheter, only surrogate data with contrast dye. An animal model can help verify this retrograde flow hypothesis.
CONCLUSION
IV-induced pneumocephalus in this study is rather uncommon, occurring in one in 3,000 head CTs ordered from the ED, but accounts for one of 20 cases of pneumocephalus in this retrospective study. IV injection of air should be considered in the evaluation of unexplained pneumocephalus in the ED by noting the absence of pathological findings on history and physical examination and identifying the characteristic radiographic air pattern on head CTs. Awareness of the clinical entity of IV-induced pneumocephalus can help improve the care for patients presenting with unexplained pneumocephalus in the ED.
Footnotes
The authors would like to thank Kylene A. Carney, MD and Kelly S. Schroeder, MD for their help in collecting the data.
Supervising Section Editor: Kurt R Denninghoff MD
Submission history: Submitted April 26, 2009; Revision Received September 9, 2009; Accepted October 29, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: T. Paul Tran, MD, University of Nebraska Medical Center, Department of Emergency Medicine, 981150 Nebraska Medical Center, Omaha, NE 68198-1150 Voice: 402-559-2571
Email: ttran@unmc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Counselman FL, Derkum S. An unusual cause of altered mental status. J Emerg Med.1995;13(6):757–63. [PubMed]
2. Jelsma F, Moore DF. Cranial aerocele. Am J Surg. 1954;87:437–51. [PubMed]
3. Dandy WE. Pneumocephalus (Intracranial Pneumatocele or Aerocele) Archives of Surgery.1926;12(5):949–82.
4. Chan YP, Yau CY, Lewis RR, et al. Acute confusion secondary to pneumocephalus in an elderly patient. Age Ageing. 2000;29(4):365–7. [PubMed]
5. Markham JW. The clinical features of pneumocephalus based upon a survey of 284 cases with report of 11 additional cases. Acta Neurochir (Wien) 1967;16(1):1–78. [PubMed]
6. Goldmann RW. Pneumocephalus as a consequence of barotrauma. JAMA. 1986;255(22):3154–6.[PubMed]
7. Malik K, Hess DC. Evaluating the comatose patient. Rapid neurologic assessment is key to appropriate management. Postgrad Med. 2002;111(2):38–6. 49. [PubMed]
8. Heckmann JG, Ganslandt O. Images in clinical medicine. The Mount Fuji sign. N Engl J Med.2004;350(18):1881. [PubMed]
9. Bartholomeus MG, de Leeuw FE. Neurological picture. “Bubbling brain” J Neurol Neurosurg Psychiatry. 2008;79(6):671. [PubMed]
10. Reddy HV, Queen S, Prakash D, et al. Tension pneumocephalus: an unusual complication after lung resection. Eur J Cardiothorac Surg. 2003;24(1):171–3. [PubMed]
11. Michel L, Khanh NM, Cedric B, et al. Air in “extra-dural space” after hyperbaric oxygen therapy. J Trauma. 2007;63(4):961. [PubMed]
12. Hyam JA, Morgan L, Mendoza ND. Coma caused by spontaneous otogenic pneumocephalus. Clin Neurol Neurosurg. 2008;110(1):62–4. [PubMed]
13. Babl FE, Arnett AM, Barnett E, et al. Atraumatic pneumocephalus: a case report and review of the literature. Pediatr Emerg Care. 1999;15(2):106–9. [PubMed]
14. Ishiwata Y, Fujitsu K, Sekino T, et al. Subdural tension pneumocephalus following surgery for chronic subdural hematoma. J Neurosurg. 1988;68(1):58–61. [PubMed]
15. Hyam JA, Morgan L, Mendoza ND. Coma caused by spontaneous otogenic pneumocephalus. Clin Neurol Neurosurg. 2008;110(1):62–4. [PubMed]
16. Gore PA, Maan H, Chang S, et al. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg. 2008;108(5):926–9. [PubMed]
17. Goldmann RW. Pneumocephalus as a consequence of barotrauma. JAMA. 1986;255(22):3154–6.[PubMed]
18. Jantzen JP. Prevention and treatment of intracranial hypertension. Best Pract Res Clin Anaesthesiol. 2007;21(4):517–38. [PubMed]
19. Bhatoe HS. Missile injuries of the anterior skull base. Skull Base. 2004;14(1):1–8.[PMC free article] [PubMed]
20. Fix A, Lang VJ. A complication of forceful nose-blowing. Am J Med. 2007;120(4):328–9.[PubMed]
21. Pitt TT. Intracranial aerocele in facial injury. Med J Aust. 1982;1(12):499–502. [PubMed]
22. Jarjour NN, Wilson P. Pneumocephalus associated with nasal continuous positive airway pressure in a patient with sleep apnea syndrome. Chest. 1989;96(6):1425–6. [PubMed]
23. Thompson TP, Levy E, Kanal E, et al. Iatrogenic pneumocephalus secondary to intravenous catheterization. Case report. J Neurosurg. 1999;91(5):878–80. [PubMed]


